Carbonyl Reactions for the DAT
/Learn key DAT concepts related to carbonyl reactions, plus practice questions and answers
Everything you need to know about carbonyl reactions for the dat
----
Part 1: Introduction to carbonyl reactions
Carbonyl reactions are at the heart of many processes that shape our daily lives, from the synthesis of pharmaceuticals to the flavors in our food. These reactions, involving compounds with a carbon-oxygen double bond, are incredibly versatile and essential in organic chemistry. Whether you're understanding how your body metabolizes sugars, how perfumes are crafted, or how new materials are designed, carbonyl chemistry plays a crucial role. This guide will cover everything you need to know about carbonyl reactions for the DAT. Pay attention to high-yield terms, and test your knowledge with DAT-style practice questions and answers at the end.
----
Part 2: Fundamentals of carbonyl reactions
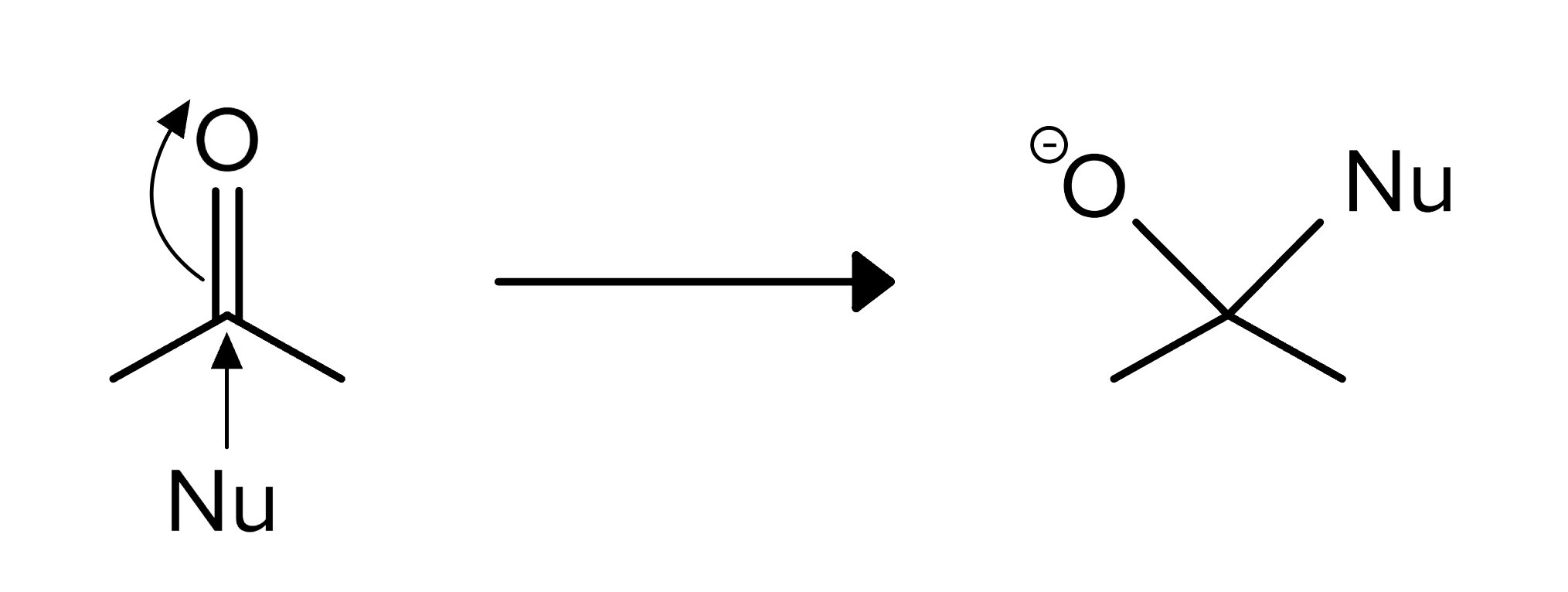
Carbonyl compounds are characterized by a carbon-oxygen double bond (C=O). They are central to organic chemistry due to their versatility and reactivity. The carbonyl group is highly polarized, with the carbon atom being electrophilic and the oxygen atom being nucleophilic. This polarity makes carbonyl compounds susceptible to a wide range of nucleophilic attack reactions. In nucleophilic addition reactions, nucleophiles, such as hydrides or alkoxides, attack the electrophilic carbon, breaking the double bond and forming a tetrahedral intermediate. This mechanism is fundamental in the formation of alcohols from aldehydes and ketones.
FIGURE 1: CARBONYL REACTION BASIC MECHANISM
Another key reaction type is nucleophilic acyl substitution, which is predominant in carboxylic acid derivatives like esters, amides, and anhydrides. Here, a nucleophile replaces the leaving group attached to the carbonyl carbon, facilitating the transformation between different derivatives. For instance, an ester can be converted into an amide by the nucleophilic attack of an amine, displacing the alcohol group. Additionally, carbonyl compounds can undergo alpha-substitution reactions due to the acidity of alpha-hydrogens, enabling enolate formation and subsequent reactions such as aldol condensations. These fundamental reactions underscore the importance of understanding carbonyl chemistry in synthesizing and transforming organic compounds.
----
Part 3: Aldehyde and ketone reactions
Aldehydes and ketones are functional groups characterized by the presence of R-CHO and R2C=O groups, respectively. Here, "R" represents a generic placeholder for the rest of the molecule. These groups are significant due to their electrophilic nature, where the oxygen atom pulls electron density away from the carbon, creating a partial positive charge on the carbon atom. This makes the carbon atom a prime target for nucleophilic attack.
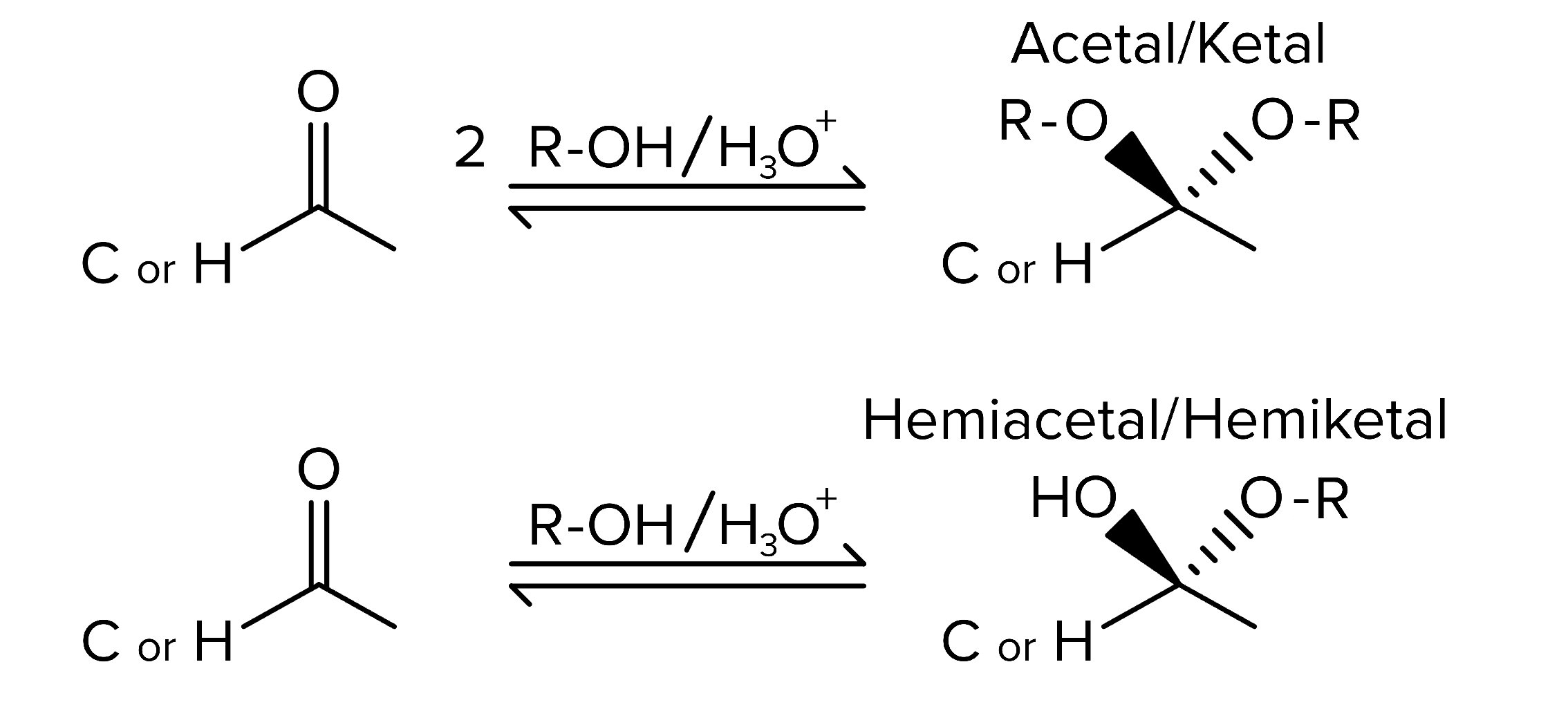
Both aldehydes and ketones frequently participate in nucleophilic addition reactions. To protect aldehydes and ketones from unwanted reactions, they can be converted into ketals/hemiketals or acetals/hemiacetals. Ketals form when ketones react with two equivalents of an alcohol, while hemiketals result from one alcohol equivalent. Similarly, acetals and hemiacetals are derived from aldehydes under the same conditions. These reactions are reversible, making them effective for protecting the electrophilic carbonyl carbon.
FIGURE 2: FORMATION OF KETALS/HEMIKETALS AND ACETALS/HEMIACETALS
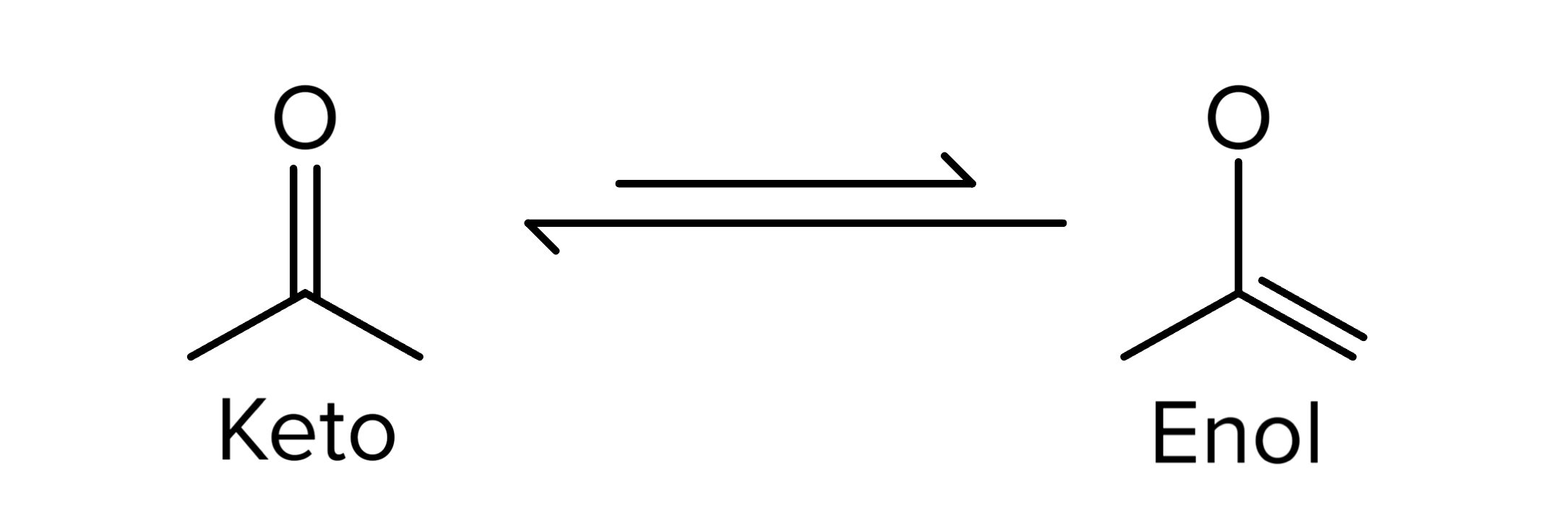
Aldehydes are also prone to oxidation reactions, thus converting them into carboxylic acids. These reactions are similar to how alcohols are oxidized to aldehydes or ketones. For a review of alcohol oxidation reactions, see our guide on additional reactions. This transformation increases the oxidation state of the functional group. Moreover, the addition of a base or acid to a ketone can lead to the formation of enolates or enols, respectively, due to the unusually acidic nature of alpha hydrogens. This process, known as keto-enol tautomerism, highlights the dynamic nature of these compounds. Ketones are electrophilic and more stable than enols, which are nucleophilic. As such, the keto form is more common.
FIGURE 3: KETO-ENOL TAUTOMERIZATION
The reactivity of aldehydes and ketones is influenced by nearby substituents. Electron-donating groups can decrease reactivity, while electron-withdrawing groups can enhance it. Steric hindrance also plays a role; aldehydes located at the ends of molecules are more accessible than ketones, which may be hindered by surrounding carbon atoms and substituents.
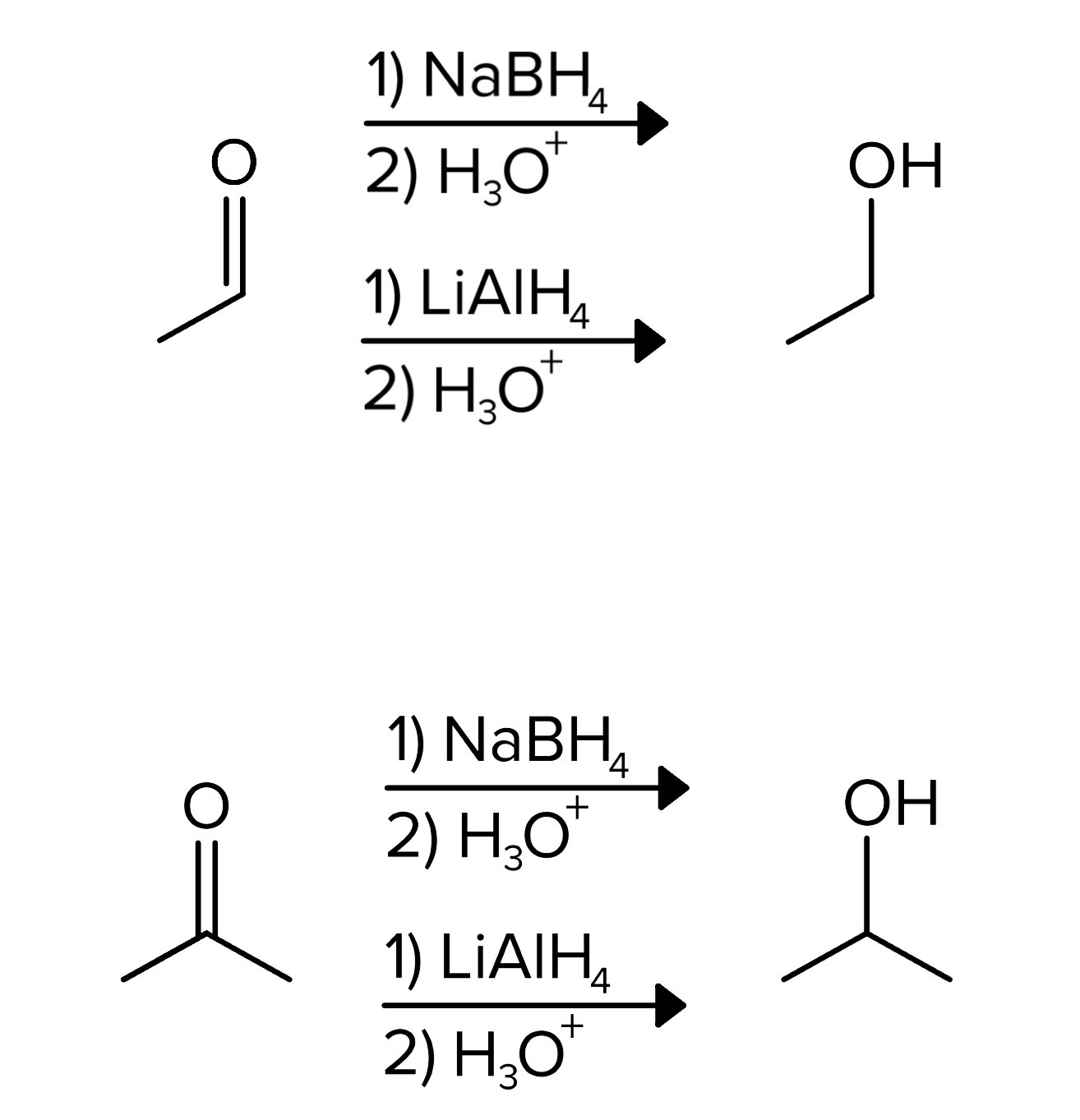
An important example is the formation of alcohols, where hydride donors such as sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4) reduce ketones to secondary alcohols. These reactions follow the same mechanism as most carbonyl reactions. A hydrogen atom from NaBH4 or LiAlH4 attacks the double-bonded carbon, forming a tetrahedral intermediate. The electrons from the double bond move to the oxygen atom, creating a negative charge. These electrons then attack a hydrogen atom from an acid, thus forming the alcohol in the product.
FIGURE 4: KETONE/ALDEHYDE REDUCTION REACTIONS
Ketones also participate in nucleophilic addition-elimination reactions, particularly when forming derivatives such as imines and enamines. In the presence of primary amines, ketones undergo condensation to form imines (Schiff bases), which are useful intermediates in various synthetic pathways. Secondary amines react with ketones to form enamines, which are valuable in many C-C bond-forming reactions. Although you don’t need to know the mechanism of these reactions, you should know the reactants, reagents, and products.
FIGURE 5: ENAMINE AND IMINE FORMATION
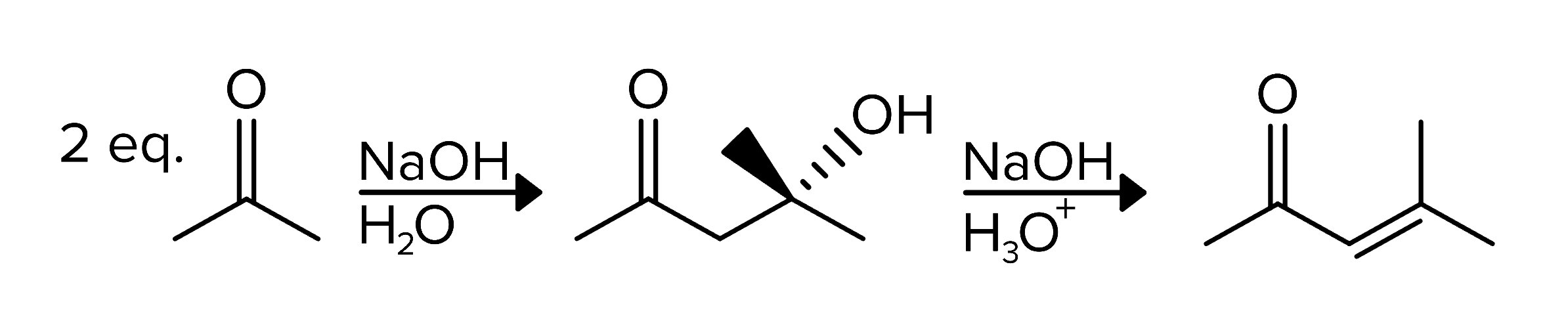
Another significant reaction involving ketones is the aldol reaction, which is pivotal in forming carbon-carbon bonds. In the presence of a base, ketones can form enolate ions, which then attack another carbonyl compound to produce a β-hydroxy ketone (aldol). This product can undergo further dehydration to yield an α,β-unsaturated ketone. The reverse process, the retro-aldol reaction, breaks down these compounds back into the original ketone and aldehyde components.
FIGURE 6: ALDOL REACTION
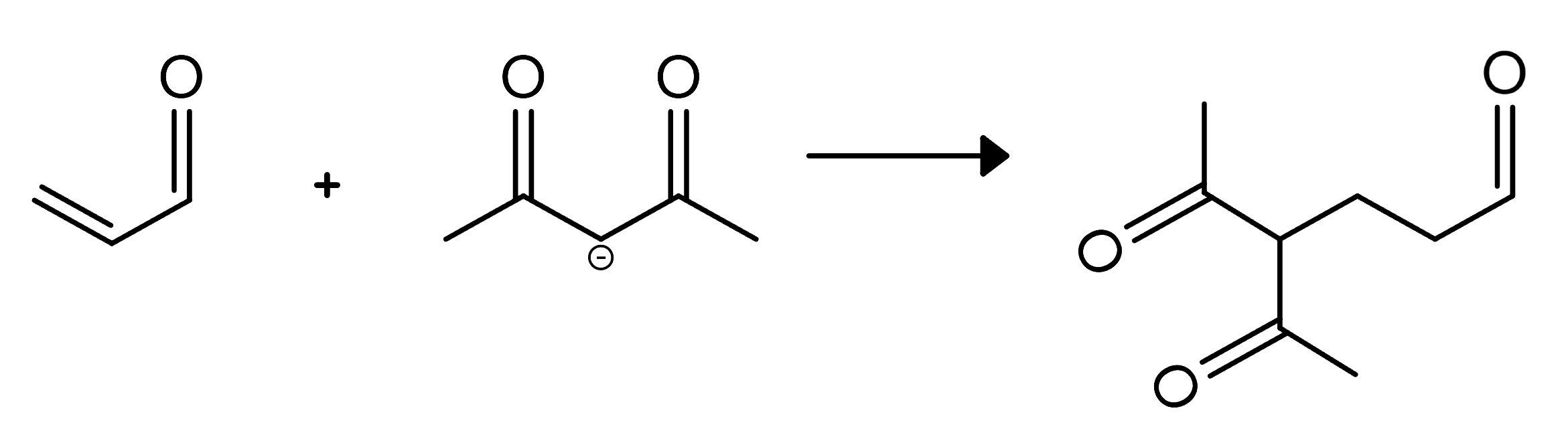
Additionally, the acidity of alpha hydrogens in ketones allows for the formation of enolates, which are crucial intermediates in many synthetic reactions. Enolates can undergo a variety of transformations, including alkylation and acylation, to form more complex molecules. This property is harnessed in reactions like the Claisen condensation (covered in additional reactions guide), where esters and ketones react to form β-keto esters, and the Michael addition, where enolates add to α,β-unsaturated carbonyl compounds.
FIGURE 7: MICHAEL ADDITION REACTIONS
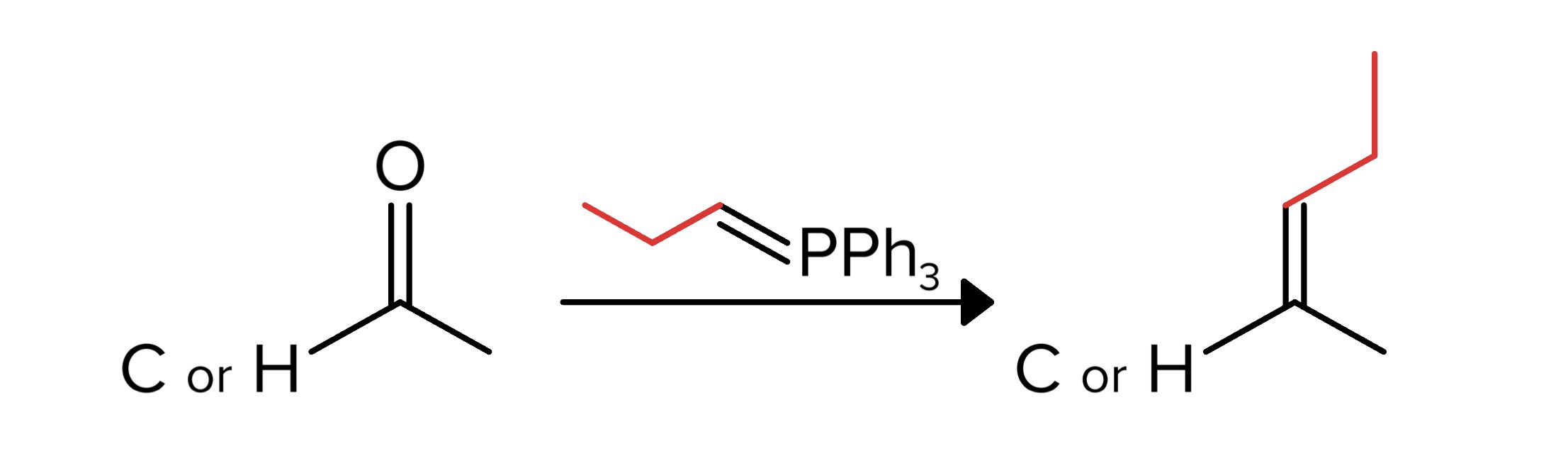
Another aldehyde/ketone reaction to know is the Wittig reaction. In this reaction, a compound known as an ylide reacts with an aldehyde or ketone to produce an alkene. Whatever is double bound to the -PPh3 group on the ylide is found in the place of the oxygen on the reactant. While you don’t need to know the mechanism for this reaction, you should know that it involves a tetrahedral intermediate.
FIGURE 8: WITTIG REACTION
----
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.