Substitution and Elimination Reactions for the DAT
/Explore high-yield DAT substitution and elimination reaction topics, with guided practice questions and explanations to boost understanding and performance.
Everything you need to know about substitution and elimination reactions for the dat
Table of Contents
Part 1: Introduction to substitution and elimination
Part 2: Fundamentals
a) Leaving groups
b) Alkyl halides
Part 3: Substitution
a) Sn2 reactions
b) Sn1 reactions
Part 4: Elimination
a) E2 reactions
b) E1 reactions
Part 5: High-yield terms
Part 6: Questions and answers
----
Part 1: Introduction to substitution and elimination
In this guide, you’ll learn to master two types of reactions: substitution and elimination. These reactions are fundamental to organic chemistry. Before diving into the complexities of these reactions, understand that substitution involves a nucleophile attacking an electrophilic center and replacing a leaving group. On the other hand, elimination involves removal of a proton and a leaving group, forming a double bond in the process. You will also learn about the mechanisms behind these reactions, which may help you better understand the general way in which organic compounds interact with each other previously learned reactions. For a quick refresh on this topic, review part 3 of the fundamentals guide. You’ll also want to review the substitution and elimination mechanisms in the same guide before moving further into this guide. Test your knowledge with practice questions and answers at the end of this guide.
----
Part 2: Fundamentals
a) Leaving groups
Before diving too far into substitution and elimination, you should understand how leaving groups relate to these reactions. Good leaving groups are essential to them, increasing the probability that these reactions will occur. Without a good leaving group, a substitution or elimination reaction will not take place. Good leaving groups tend to be weak bases, whereas bad leaving groups are strong bases. Below are lists of good and bad leaving groups you may find on the DAT. Note that alkyl groups are bad leaving groups. This is constant for all hydrocarbons.
FIGURE 1: GOOD AND BAD LEAVING GROUPS
Spotting a good leaving group is a skill you should master for the DAT. Good leaving groups are sp3 hybridized and commonly halogens. For a review of hybridization, see our guide on bonding and aromatics. Even if a good leaving group, like a halogen, is present, remember that without a good leaving group, you should not expect substitution or elimination to occur.
FIGURE 2: EFFECT OF HYBRIDIZATION ON LEAVING GROUPS
b) Alkyl halides
Another skill that will help you with substitution and elimination reactions is being able to identify alkyl halides as primary (1°), secondary (2°), and tertiary (3°). To determine this, simply count the number of carbon or other non-hydrogen atoms connected to the halogen-bound carbon. Because halogens make good leaving groups, alkyl halides are often an indicator of substitution or elimination.
FIGURE 3: CLASSIFYING ALKYL HALIDES
Now that you’ve reviewed the concepts above, you’re ready to dive into substitution and elimination. Fundamentally, there are two types of substitution (Sn1 and Sn2) and two types of elimination (E1 and E2). We will cover each individually, and follow that with practice in distinguishing between them. As we go through the different reaction types, pay attention to the similarities and differences between them to better prepare for the challenge of deciphering between reaction types, as these questions are common on the DAT.
----
Part 3: Substitution
a) Sn2 reactions
In an Sn2 reaction, ‘S’ stands for substitution, ‘N’ stands for nucleophilic, and ‘2’ specifies that the reaction is bimolecular. The term ‘biomolecular’ refers to the rate-determining step of a reaction, the step in which the nucleophile and electrophile interact. This is a back-side attack in Sn2 reaction due to the front being blocked by the leaving group. This nucleophilic attack results in stereochemical inversion, shown in the visual below. Essentially, if the configuration started out as R, an Sn2 reaction will flip it to S (and vice versa).
Because the back-side attack is rate-determining, the speed of this single step determines the speed of the reaction. Look below for a visual of the Sn2 reaction mechanism. Another factor determining whether a substitution reaction can occur in one step is the strength of the nucleophile involved. Stronger nucleophiles are prone to undergo the Sn2 reaction with their substrate.
FIGURE 4: MECHANISM OF SN2 REACTIONS
Several factors influence the speed at which Sn2 reactions can occur. First, consider steric hindrance. The more substituted (bulky) a molecule is at the point of attack, the less reactive it will be. Following this trend, you can infer that methyl groups have the greatest reactivity, followed by primary and secondary haloalkanes. Most Sn2 reactions involve methyl groups or primary substitution. Tertiary compounds cannot undergo the Sn2 mechanism.
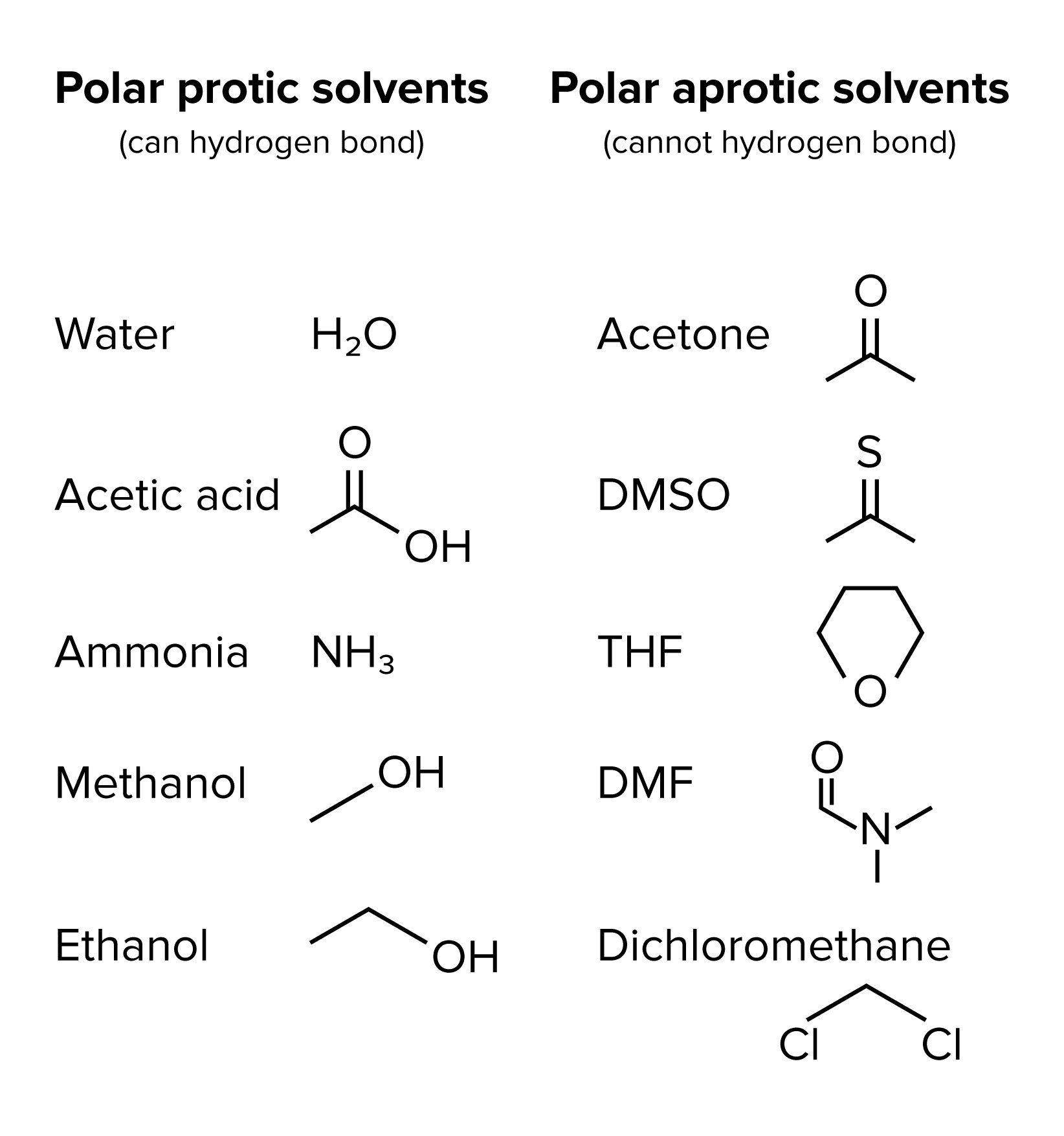
The final factor to consider when determining whether a substitution or elimination reaction will occur, as well as whether that reaction will be a one or two-step reaction, is the solvent. Polar aprotic solvents favor one-step reactions (Sn2 and E2) because they solvate the electrophile without excessively solvating the nucleophile. On the other hand, polar protic solvents promote carbocation stability, thus favoring two-step reactions (Sn1 and E1). Aprotic solvents do not engage in hydrogen bonding, whereas protic solvents do. You’ll benefit from becoming familiar with the list of common polar aprotic solvents shown below.
FIGURE 5: POLAR PROTIC AND POLAR APROTIC SOLVENTS
If you’re keeping track of the factors differentiating substitution and elimination, items to pay attention to include leaving group, steric hindrance, strength of the nucleophile/base, solvent, and stereochemistry. Incorporating each of these factors into deciding the type of reaction taking place can be overwhelming at first. To become more comfortable with these problems for the DAT, you’ll benefit from doing plenty of practice problems and considering the corresponding explanations.
----
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.