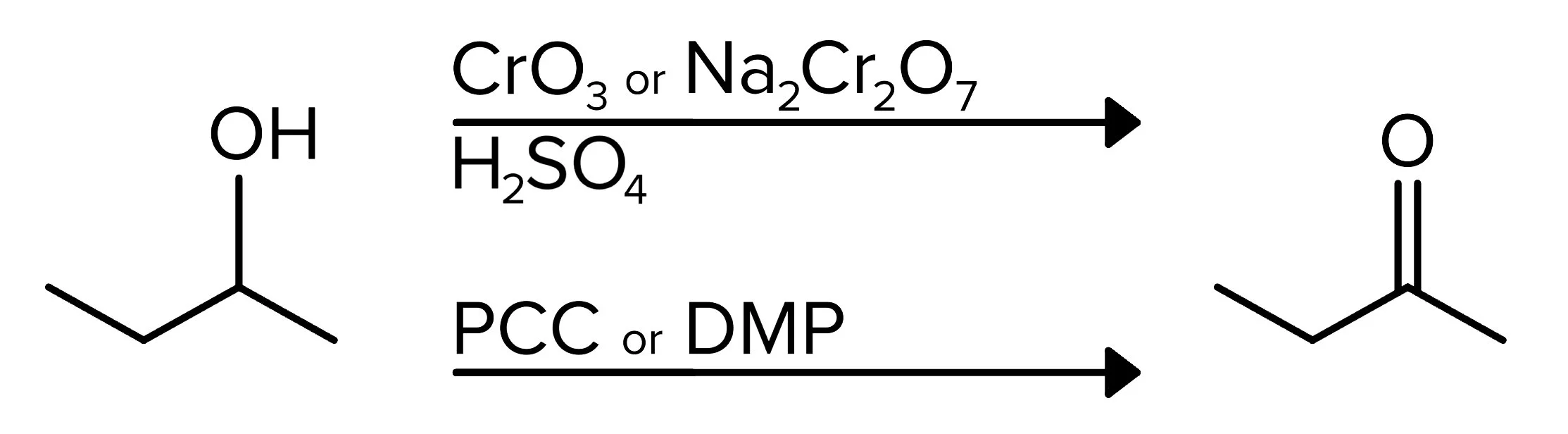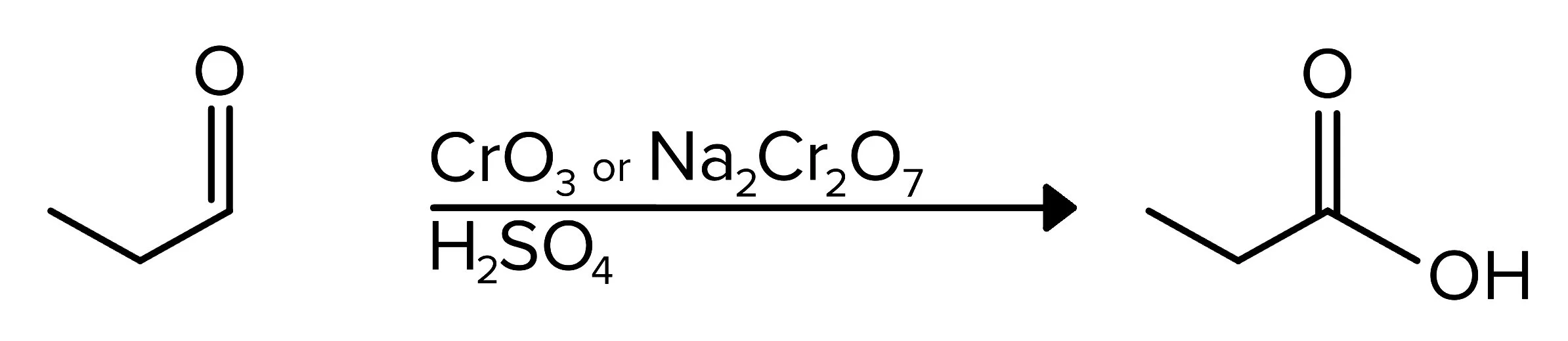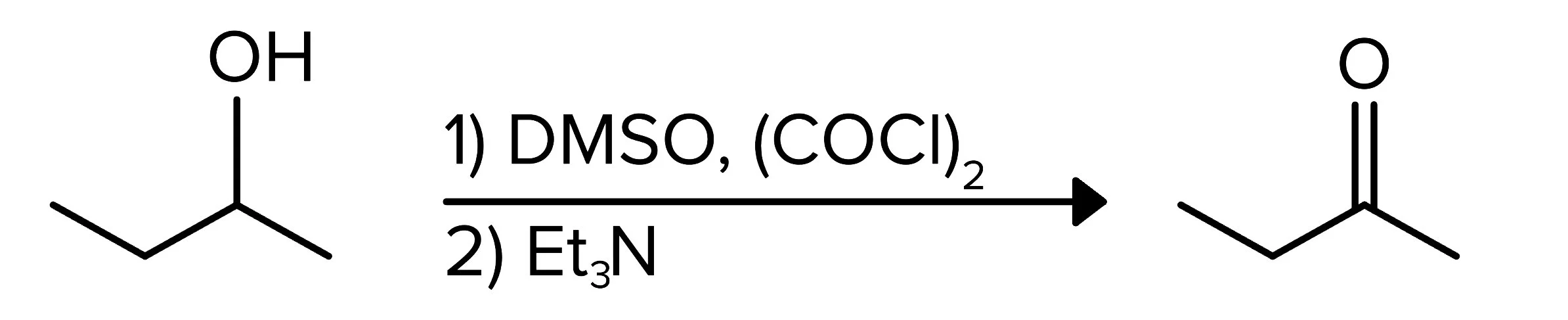Additional Reactions for the DAT
/Learn everything you need to know about alcohol, ether, epoxide, nitrile, alpha-carbon, and Diels-Alder organic reactions for the DAT, plus practice questions and answers
Learn everything you need to know about additional reactions for the dat
Table of Contents
Part 1: Introduction to additional reactions
Part 2: Alcohol reactions
a) Alcohol to halide reactions
b) Oxidation reactions
c) Other alcohol reactions
Part 3: Epoxide and ether reactions
a) Epoxide reactions
b) Ether reactions
Part 4: Nitrile reactions
Part 5: Alpha-carbon reactions
a) Alpha halogenation
b) Ester synthesis
c) Condensation reactions
Part 6: Diels-Alder reactions
Part 7: High-yield terms
Part 8: Questions and answers
----
Part 1: Introduction to additional reactions
The DAT requires you to know A LOT of reactions. Even though we’ve covered many reactions in previous guides, there are still more you need to know. This guide will walk you through reactions related to alcohols, epoxides, ethers, nitriles, and more. On the DAT, it will be difficult to deduce products for reactions you haven’t studied, so we recommend memorizing the reactants, reagents, and products for these and all other reactions. Once you’ve studied them, practice with DAT-style questions and answers at the end of this guide.
----
Part 2: Alcohol reactions
a) Alcohol to halide reactions
Alcohol groups (-OH) play a role in many reactions you need to know for the DAT. In the first set of reactions, the alcohol group is removed and a halide is added via a substitution mechanism. This reaction is often done in order to change the alcohol, a poor-leaving group, into a better leaving group for subsequent reactions.
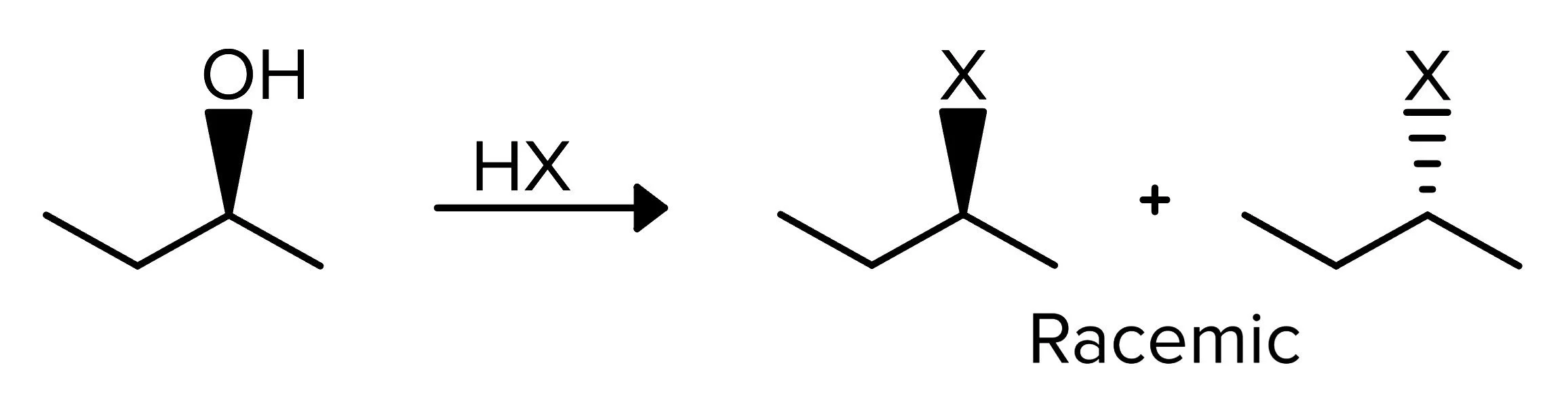
The first reaction works for 2° or 3° alcohols and uses an Sn1 mechanism. Because the mechanism is Sn1, rearrangements can happen. A compound with an alcohol group is reacted with a halide consisting of a hydrogen and a halogen (X), typically fluorine, chlorine, bromine, or iodine. Because this is an Sn1 reaction, there will be a racemic mixture of products.
FIGURE 1: 2° ALCOHOL TO HALIDE USING HX
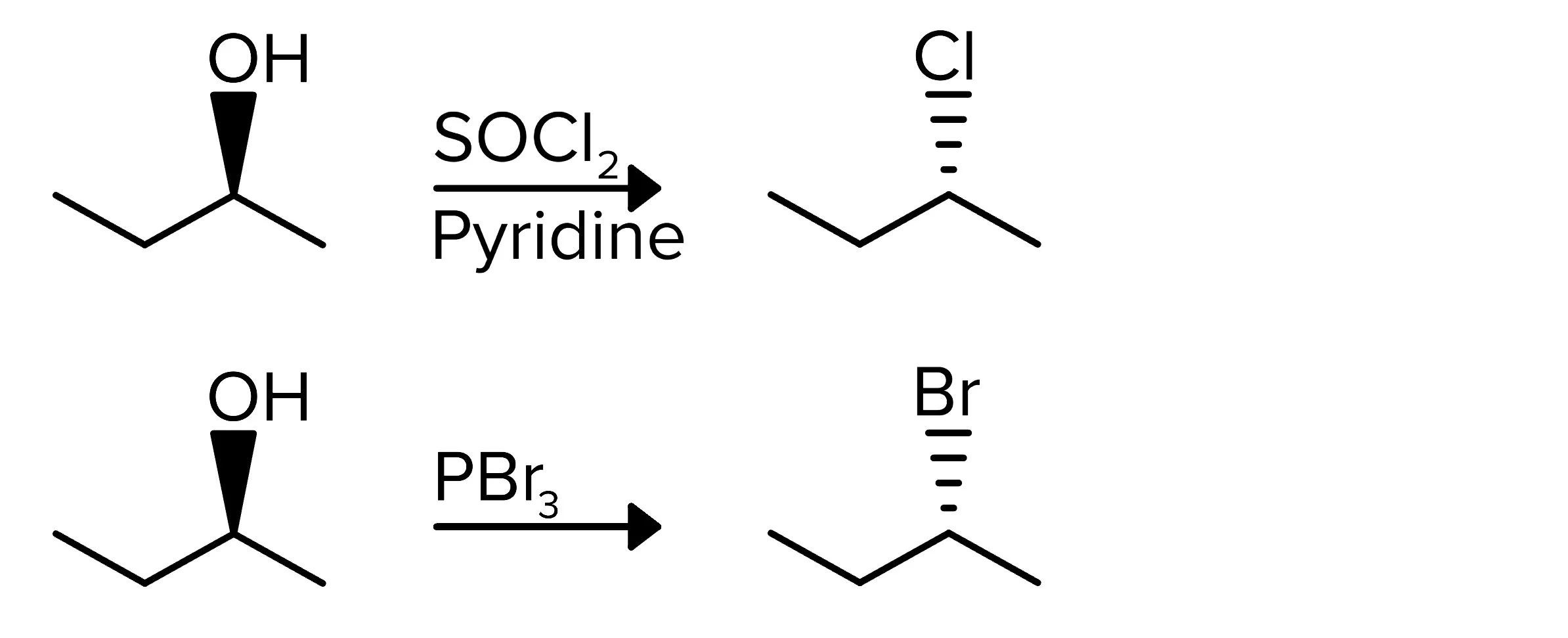
The following two reactions achieve similar end products to the previous reaction, but they follow an Sn2 mechanism. These reactions work for 1° and 2° alcohols. In the first reaction, SOCl2 and pyridine add a chlorine atom in place of the OH. In the second reaction, PBr3 is used to substitute the alcohol group with a bromyl group. Remember that stereochemistry is inverted because of the Sn2 mechanism.
FIGURE 2: 1° OR 2° ALCOHOL TO HALIDE USING USING SOCL2 OR PBR3
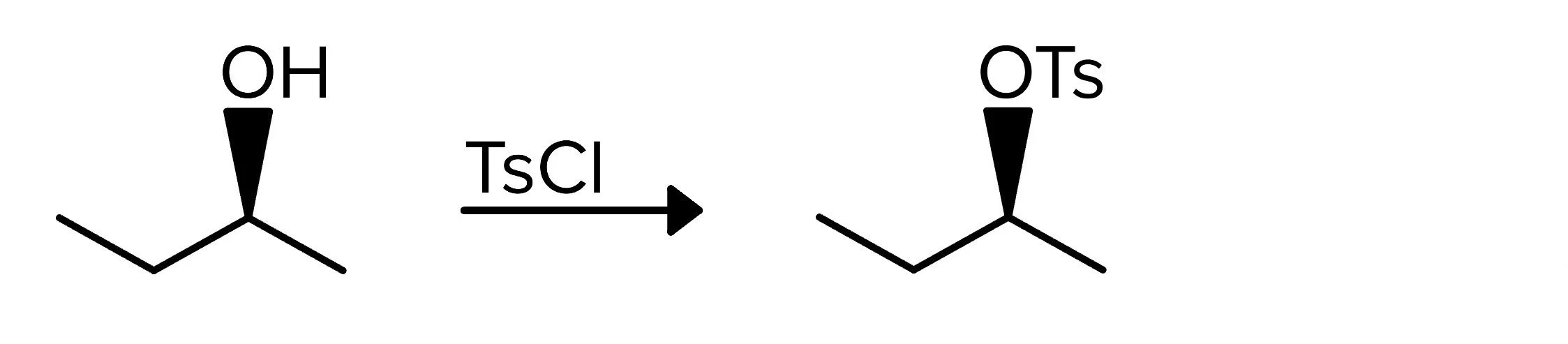
The next reaction doesn’t entirely replace the alcohol group, but it does substitute the hydrogen atom in order to form a better leaving group. A compound with an alcohol is reacted with a compound containing a tosyl group, abbreviated Ts. This tosyl group takes the place of the hydrogen atom to form a tosylate ester (OTs), a good leaving group. In this reaction, stereochemistry does not change.
The mechanism for this reaction involves the oxygen from the alcohol attacking the sulfur atom in the tosyl chloride compound, and the chloride atom is removed and forms an ion. In the following step, the chloride ion attacks and removes the hydrogen atom from the original -OH group, forming HCl and leaving the oxygen and its attached tosyl group.
FIGURE 3: FORMATION OF A TOSYLATE ESTER USING TOSYL CHLORIDE
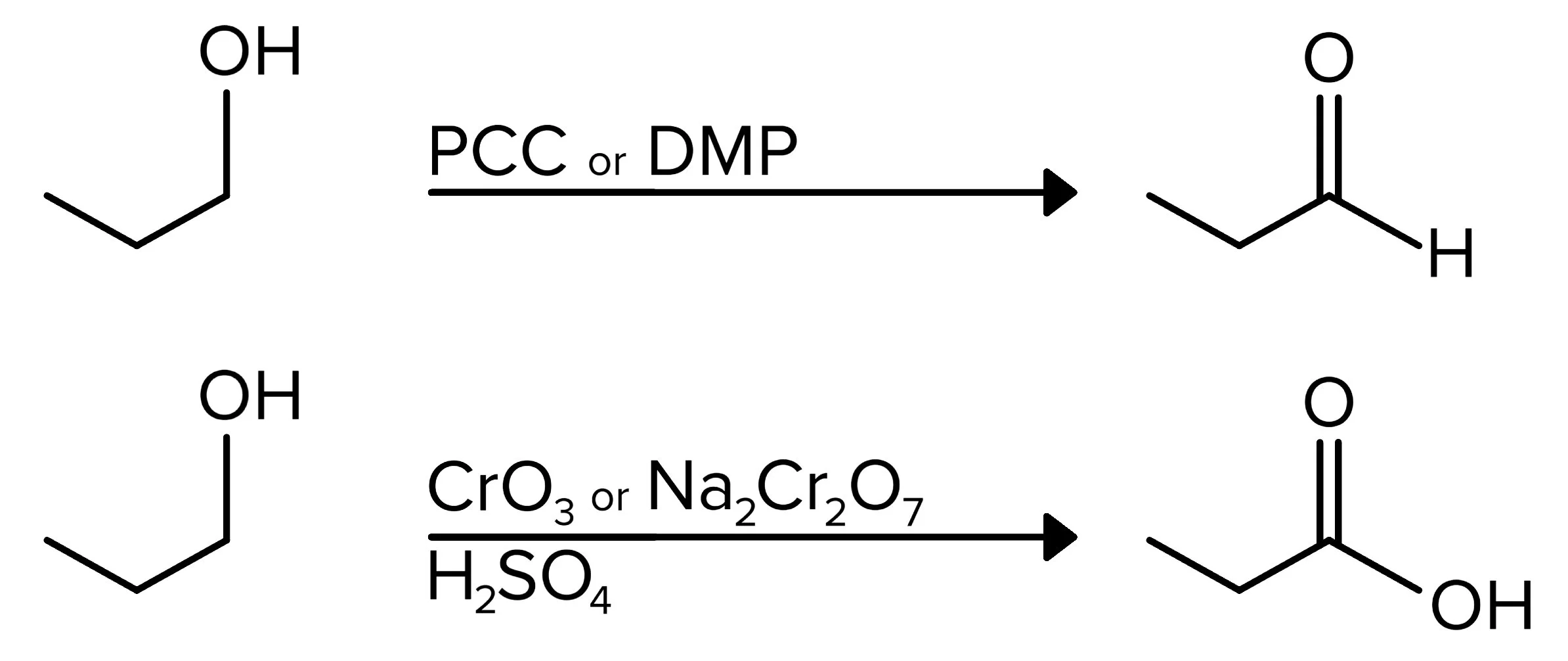
b) Oxidation reactions
The next set of alcohol reactions result in the reactant being oxidized. In these reactions, the hydrogen in the alcohol group is removed, and a double bond is formed between oxygen and carbon. Because this carbon atom is now double bonded to the oxygen instead of single bonded, it has been oxidized.
The first type of oxidation reactions applies to 2° alcohols. When reacted with a reagent containing chromium (CrO3 or Na2Cr2O7) as well as a strong acid (H2SO4 is used below), a carbonyl group is formed. The mechanism for this reaction takes place in three steps. In the first step, the oxygen in the OH group attaches to chromium reagent, and one of the oxygen atoms in this reagent removes a hydrogen from H2SO4. The second step consists of the hydrogen attached to the OH group being removed by the conjugate base of sulfuric acid (-HSO4). Finally, a hydrogen atom on the carbon attached to the oxygen is removed, and a double bond is formed, making this the carbonyl carbon. This same result is achieved when PCC or DMP is used as a reagent.
FIGURE 4: OXIDATION OF A 2° ALCOHOL USING CHROMIC ACID OR PCC/DMP
For 1° alcohols, the same reagents can be used to produce carbonyl groups, but there is a key difference in the products. When PCC or DMP are the reagents, the reaction proceeds the same as before, leading to the formation of an aldehyde. However, when a chromium reagent is used, the reaction results in a carboxylic acid.
FIGURE 5: OXIDATION OF 1° ALCOHOL TO ALDEHYDE OR CARBOXYLIC ACID
On this same note, chromium-based reagents can oxidize an aldehyde to a carboxylic acid, as shown below.
FIGURE 6: CONVERTING AN ALDEHYDE GROUP TO A CARBOXYLIC ACID GROUP
Swern oxidation reactions represent another way to oxidize an alcohol-containing compound. These reactants work on either 1° or 2° alcohols. Similar to PCC or DMP, these reagents lead to a carbonyl group. The reagents are used in two steps, with the first step consisting of DMSO, and oxalyl chloride (COCl)2. The second step adds a triethylamine, Et3N.
FIGURE 7: SWERN OXIDATION OF AN ALCOHOL
c) Other alcohol reactions
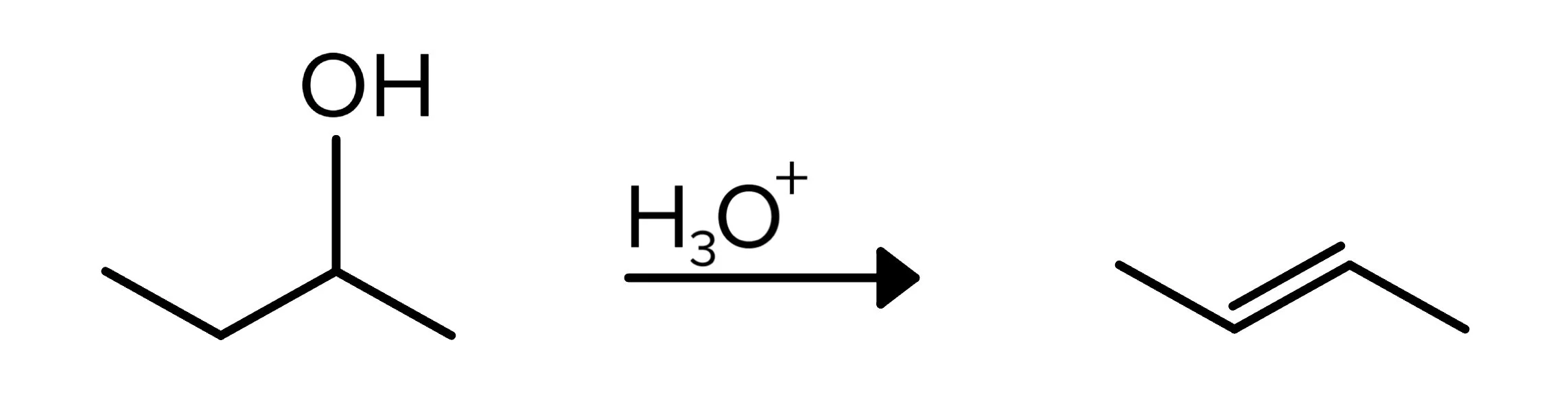
Compounds with an alcohol group can have their -OH group removed through a dehydration reaction. In this reaction, H3O+ (or another strong acid) is the reagent, and the resulting compound has a double bond. The mechanism is E2 for 1° alcohols, and E1 for 2° and 3° alcohols. The placement of this double bond follows Zaitsev’s rule. For a review of Zaitsev’s rule, see our guide on hydrocarbon reactions.
FIGURE 8: DEHYDRATION OF ALCOHOL REACTION
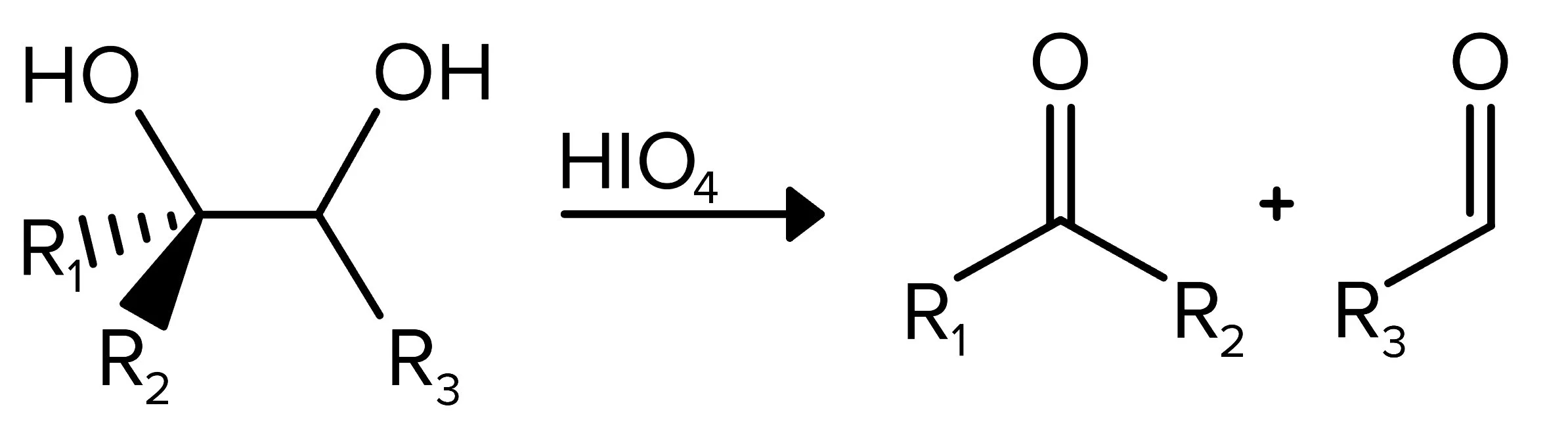
A diol is a compound with two alcohol groups separated by two carbons. Diols can be separated into two different carbonyl-containing compounds when reacted with HIO4. This reaction is known as oxidative cleavage. The resulting carbonyl compounds can be either an aldehyde or a ketone.
FIGURE 9: OXIDATIVE CLEAVAGE REACTION
----
Part 3: Epoxide and ether reactions
a) Epoxide reactions
The next type of reactions are for compounds containing an ether or epoxide functional group. Recall that epoxides are compounds that have two different carbon atoms bonded to the same oxygen atom as part of a three-membered ring.
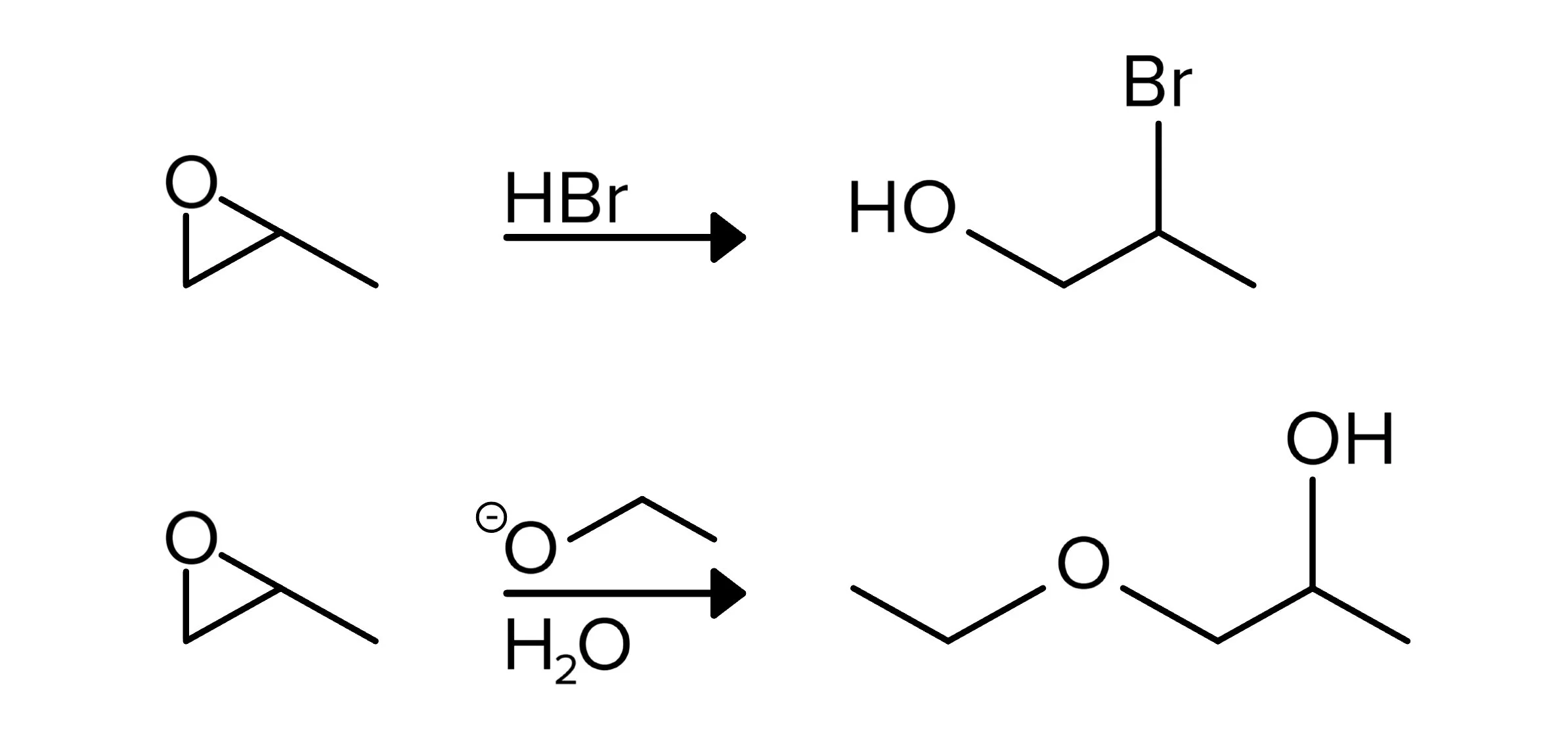
Epoxide reactions differ based on whether they react with an acid or base, with both acid and base compounds capable of opening an epoxide ring. Depending on which compound is used, the ring will open on different sides. When an acid is used, the ring will open and the nucleophile (from the acid) will attack the more substituted side. On the other hand, nucleophiles from basic compounds will attack the less substituted side. The oxygen atom is then protonated and becomes an alcohol. Although the reagents used can vary from those shown below, the concept of acids and bases opening the ring on their respective side still applies.
FIGURE 10: ACIDIC NUCLEOPHILES ATTACK TO THE MORE SUBSTITUTED SIDE (TOP REACTION) WHILE BASIC NUCLEOPHILES ATTACK TO THE LESS SUBSTITUTED SIDE (BOTTOM REACTION)
----
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.