MCAT Organic Chemistry: Everything You Need to Know
/The topics you need to know to ace MCAT organic chemistry questions, plus strategies to help you maximize your score
----
Part 1: Introduction
The MCAT is the last step between you and your white coat, and you’re getting ready to study for the longest and hardest exam of your life. The MCAT plays a huge role in medical school admissions. If you have a GPA between 3.60 and 3.79, increasing your MCAT score from a 497 to a 514 leads to a 61.6% increase in your admissions odds!
Interestingly, the number of questions asked on a certain topic on the MCAT does not correlate to how much you studied the topic in college. You likely spent one or two entire semesters studying organic chemistry, but the MCAT will ask you only about 6 to 12 questions on organic chemistry out of 230 total questions. In other words, only 3 to 5 percent of your entire exam is likely to cover organic chemistry.
Why do we mention this? Many students spend hours, days, and weeks of valuable test prep time memorizing arcane organic chemistry reactions. Instead, students should focus their time on understanding the principles behind organic chemistry so that they can apply these principles to new problems.
For example, what is a nucleophile? If the MCAT provides a new reaction that you’ve never seen before, can you identify the nucleophile that attacks the electrophile? What properties should you look for?
In this blog, we provide all of the content you need to know for the MCAT organic chemistry questions. We don’t want you to use rote memorization to learn MCAT Ochem; instead, we’ll provide a toolbox that you can use to attack many different organic chemistry problems. In addition, we have talked to C/P and B/B 132 scorers to find out study strategies you can use to get those 6 to 12 organic chemistry questions correct and earn a perfect MCAT score. Let’s get started!
----
Part 2: The MCAT organic chemistry content you need to know for the exam
Note: Clicking on any of these thumbnails will take you to a comprehensive guide on that topic.
----
Part 3: MCAT organic chemistry study strategies
In this section of the guide, we will present three high-yield study strategies you can use to handle MCAT general chemistry questions.
MCAT Organic Chemistry Tip #1: Know the structure of functional groups like the back of your hand.
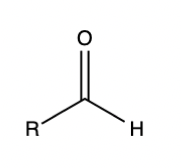
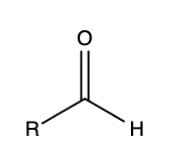
If someone asks you to draw an aldehyde, you should be able to draw it without even thinking. You should have the same level of memorization of organic chemistry functional groups as you do over amino acids. Why?
The reason functional groups are important is their structure allows them to contain interesting chemical properties. If you can draw out an aldehyde and analyze its structure, you won’t have to memorize that the carbonyl carbon is electrophilic, and the hydrogen attached the carbonyl carbon does not sterically hinder potential nucleophiles. Instead, you’ll be able to draw the functional group and quickly recall these properties by looking at the structure.
Aldehyde functional group
So, you should know what the following functional groups look like at a bare minimum:
Carbonyl
Aldehyde
Ketone
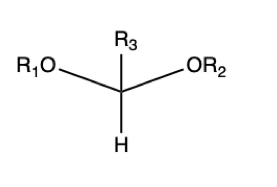
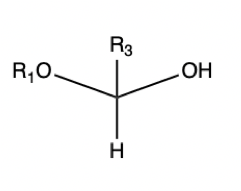
Hemiacetal
Acetal
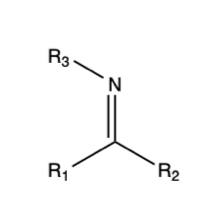
Imine
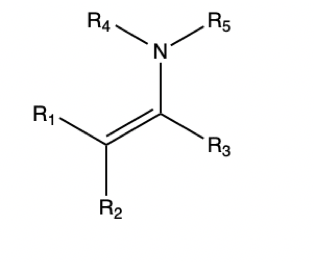
Enamine
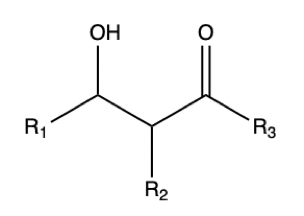
Aldol
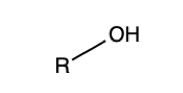
Alcohol
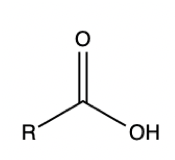
Carboxylic Acid
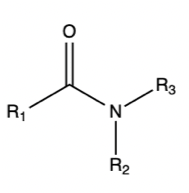
Amide
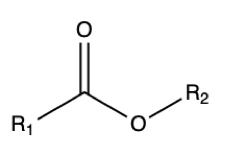
Ester
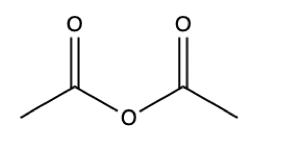
Anhydride
Phenol
MCAT Organic Chemistry Tip #2: Know the properties of functional groups so that you can understand how they will react.
Let’s look at a commonly tested example: the carboxylic acid functional group. If you draw out the structure of a carboxylic acid, you will notice that there are important parts of the molecule that contribute to how it reacts with other molecules.
For example, the oxygen double bonded to the central carbon has some interesting properties. Which is more electronegative: oxygen or carbon? If you said oxygen, that is correct! Oxygen wants electrons a lot more than carbon. As a result, the oxygen will pull on those electrons harder, causing the oxygen atom to have a partial negative charge. As a result, the carbon will have a partial positive charge.
Why is that important? It is important because the carbon is now a reactive atom. The carbon really wants electrons due to its partial positive charge, making it an electrophile. And something with an extra pair of electrons, or a nucleophile, can now attack the carbon and engage in interesting chemistry.
Understanding these properties for each functional group that we just mentioned will help you solve organic chemistry problems without having to remember specific and complicated organic chemistry reactions. Make sure to pay special attention to understanding the carbonyl functional group, which is any carbon double bonded to an oxygen, and the properties we just talked about as the MCAT loves to test students on it.
MCAT Organic Chemistry Tip #3: Understand experimental techniques commonly used in organic chemistry, such as separations, purifications, and spectroscopy.
The MCAT will expect you to be familiar with these basic organic chemistry tools, and we will walk through the details you need to know of each technique in our Ch. 5 guide on spectroscopy, separations, and purifications. Here, however, is a good place to remind you that anytime you miss an MCAT organic chemistry question while doing practice, be sure to go back and review that question, write down what you needed to know to get the question right, and study that new information. You shouldn’t just do this for practice organic chemistry questions, you should do it for all practice questions that you take.
----
Part 4: MCAT organic chemistry practice questions
1. A researcher performs an organic reaction in which a benzene with an alcohol substituent turns into a benzene with an aldehyde substituent. Which of the following peaks may appear for the product in IR spectroscopy?
A) Broad, 3500 cm-1
B) Sharp, 3500 cm-1
C) Broad, 1700 cm-1
D) Sharp, 1700 cm-1
2. Which of the following best describes why protic solvents decrease nucleophilicity?
A) Protic solvents are too sterically bulky and prevent the nucleophile from contacting the electrophile.
B) Protic solvents alter the chemical properties of the electrophile to decrease the reaction efficiency.
C) Protic solvents can hydrogen bond with the nucleophile, thereby decreasing the frequency of nucleophilic attack.
D) Protic solvents introduce a net positive charge to the nucleophile and decrease nucleophilicity.
3. A researcher is studying a molecule with three chiral centers. The researcher obtains a racemic mixture after several steps of purification. Which of the following stereochemical designations could the researcher have obtained?
A) S,S,S and R,S,R
B) R,S,S and S,R,R
C) R,S,R and S,R,R
D) S,S,R and S,S,S
4. Which of the following elements is MOST likely to be a strong nucleophile?
A) Hydroxide ion
B) Water
C) Ethanol
D) Tert-butanol
5. Which of the following standalone functional groups is most likely to undergo nucleophilic acyl substitution?
A) Primary alcohol
B) Aldehyde
C) Ketone
D) Carboxylic acid
Answer key for MCAT organic chemistry practice questions
1. Answer choice D is correct. In the product, a carbonyl group is forming (C=O), and this is seen on an IR spec at 1700 cm-1 as a sharp peak (choice D is correct). A broad peak around 3500 cm-1 is characteristic of an OH, which we would see for the reactant, not the product (choice A is incorrect).
2. Answer choice C is correct. By forming hydrogen bonds with protic solvents, nucleophiles cannot move as freely in solution and it is harder for them to attack electrophiles (choice C is correct). Protic solvents are not necessarily sterically bulky; water is an example of a protic solvent (choice A is incorrect). The question asks how protic solvents affect then nucleophile, not the electrophile (choice B is incorrect). While protic solvents might make the nucleophile less negative, they are highly unlikely to introduce a positive charge to the nucleophile (choice D is incorrect).
3. Answer choice B is correct. A racemic mixture occurs when an enantiomer pair is present in equal amounts. Enantiomers have opposite stereochemistry at every chiral center (choice B is correct; choices A, C, and D are incorrect).
4. Answer choice A is correct. Charge, electronegativity, H-bonding capacity, and steric bulk are important in determining how strong a nucleophile is. Here, hydroxide ions are negatively charged (OH-), strongly electronegative, and have no steric hindrance (choice A is correct). Water and ethanol are not very strong nucleophiles (choices B and C are incorrect). Tert-butanol has a lot of steric hindrance (choice D is incorrect).
5. Answer choice D is correct. Although carboxylic acids have a carbonyl carbon that is less electrophilic than the carbonyl carbon found in ketones and aldehydes, neither ketones nor aldehydes contain a leaving group that would depart in a nucleophilic acyl substitution reaction. (Choices B and C are incorrect.) Primary alcohols do not contain carbonyl carbons - and therefore no acyl group - thus it will not undergo nucleophilic acyl substitution. (Choice A is incorrect).