Enzymes for the MCAT: Everything You Need to Know
/Learn everything you need to know about enzymes—one of the most heavily tested science topics on the MCAT
(Note: This guide is part of our MCAT Biochemistry series.)
Part 1: Introduction to enzymes
Part 2: General enzyme characteristics
a) Catalytic activity
b) Enzyme-substrate binding
c) Types of enzymes
d) Cofactors, coenzymes, and vitamins
e) Catalytic amino acids
Part 3: Enzyme kinetics and inhibition
a) Michaelis-Menten and Lineweaver-Burk
b) Competitive inhibition
c) Uncompetitive inhibition
d) Mixed and noncompetitive inhibition
Part 4: Regulating enzyme activity
a) Local environment conditions
b) Covalent modification
c) Allosteric regulation
d) Zymogens
e) Cooperativity
f) Feedback regulation
Part 5: High-yield terms
Part 6: Enzymes practice passage
Part 7: Enzymes practice questions and answers
----
Part 1: Introduction to enzymes
Enzymes are one of the most heavily tested science topics on the MCAT, and they are an important part of our everyday life. Each cell in our body carries out many of its functions using enzymes, and misregulation of these enzymes is responsible for a wide scope of human diseases, such as cancer and hypertension.
Many students struggle with enzymes on the MCAT, often losing valuable points on questions testing topics from enzymatic inhibition to feedback regulation. In this guide, we will break down the content you need to know—no more and no less—to study enzymes for the MCAT. All of the terms bolded throughout the guide will be defined in Part 5 of the guide, but we encourage you to create your own definitions and examples as you move through this resource so that they make the most sense to you!
In addition to knowing the content, you will also need to know how to analyze the various graphs, equations, and terms related to enzymes that the MCAT presents. At the end of this guide, there is an MCAT-style enzyme practice passage and standalone questions that will both test your knowledge and show you how the AAMC likes to ask questions.
Let’s get started!
----
Part 2: General enzyme characteristics
a) Catalytic activity
Enzymes are biological catalysts, and a catalyst is defined as a substance that speeds up the rate of a chemical reaction without being consumed itself. For example, let’s say you need to get from point A to point B, and they are 10 miles apart. You could walk, which would likely take a while. Or you could drive a car and arrive a lot faster. In this case, the car serves as our catalyst. Note, the distance is not changing, but the speed with which you get there is faster.
Figure: a catalyst is a substance that speeds up the rate of a chemical reaction, in the same way that a car helps you get from point a to point b faster
In the same way that a car speeds up our transportation, an enzyme serves to speed up the rate of a biochemical reaction. As a result, an enzyme is classified as a biological catalyst, which has the following properties (we will dive into each one):
Lowers activation energy of a reaction
Affects the kinetics of the reaction, but not the thermodynamics (∆G) or equilibrium constant
Regenerates itself
Let’s first look at activation energy. Which of the following requires us to put in more energy: walking or driving 10 miles? If you said walking, you are correct. (In a car, all we have to do is press the gas pedal!) An enzyme—the car in our example—reduces the amount of input energy necessary to undertake a chemical reaction. By lowering the input energy, or activation energy, of a reaction, the reaction can occur much more quickly. This brings us to reaction kinetics.
An enzyme affects the kinetics of a reaction by speeding up the rate of the reaction. Note, the reaction rate is the rate at which reactants are consumed or the rate at which products are made. Importantly, enzymes DO NOT affect the thermodynamics (∆G) or equilibrium constant of a reaction.
Let’s look at another example to illustrate this point. We have a machine that converts red spheres into red cubes. Normally, if you give the machine 10 red spheres, it will produce 5 red cubes in 1 hour. Now, let’s say a catalyst acts on the machine—if you give the machine 10 red spheres, it will produce 5 red cubes in 1 minute.
The kinetics of the machine are affected as it can now work faster, but the thermodynamics and equilibrium constant stay the same. In each case, 10 red spheres are made into 5 red cubes, regardless of how fast the machine can do it.
We’ve illustrated these effects below in a commonly drawn reaction diagram:
Figure: A free energy versus reaction progress plot showing the effect of a catalyst on activation energy for a reaction
Finally, enzymes are regenerated during their catalytic cycles. This is very important as regeneration reduces the amount of protein that a cell has to make in order to carry out biochemical reactions. For example, cells use a lot of ATP, and most of this ATP is generated by ATP synthase, an enzyme. (As a brief aside, enzymes often end with an -ase!) If each ATP synthase could only synthesize one ATP molecule, the cell would be overrun by the ATP synthase enzymes. The ability of each ATP synthase—and other enzymes—to reset itself after each cycle is absolutely essential to our survival.
In the figure below, we show you how regeneration would make a great difference in the number of ATP synthases needed for a cell if it could not regenerate itself for multiple catalytic cycles.
Figure: why enzyme regeneration is important
b) Enzyme-substrate binding
We’ve talked about the general properties of enzymes, and now we will dip our toes into more of the nitty-gritty details. We often talk about chemical reactions by writing out reaction schemes such as A → B → C. For enzymes, we can draw out a reaction scheme that can help us better understand what exactly is going on, and it looks something like the following:
E + S → ES → E + P
E = enzyme
S = substrate
P = product
We’ll come back to the overall reaction scheme when we look at enzyme inhibition in the next section, but let’s zoom into the ES, or enzyme-substrate complex.
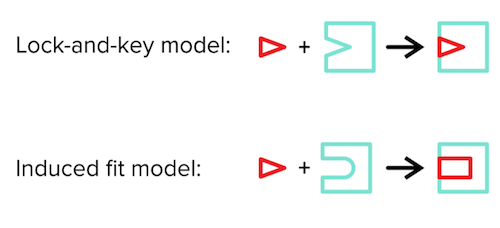
There are two models for describing the enzyme-substrate complex: 1) the lock-and-key model and 2) the induced fit model. The lock-and-key model describes the substrate as the “key” and the enzyme as the “lock.” Without changing any conformations, the key should fit nicely into the lock for the two to bind.
The induced-fit model (which is generally the more accepted model) states that enzyme and substrate conformations do not have to be as rigid as suggested by the lock-and-key model. Instead, upon substrate binding to the enzyme, both will undergo slight conformational changes to improve their binding to one another.
Figure: lock and key model vs induced fit model
c) Types of enzymes
There are six well-known types of enzymes that the MCAT wants you to know:
| Enzyme type | Function | Example |
|---|---|---|
d) Cofactors, coenzymes, and water-soluble vitamins
Most enzymes require small ions or proteins to help them function properly. Cofactors/coenzymes usually bind to the enzyme’s active site and assist in catalyzing the reaction. The two are essentially the same, except coenzymes are proteins whereas cofactors can be ions, such as Mg2+. Water-soluble vitamins may also play roles in enzymatic activity, and they have to be obtained from our diets.
If the cofactor or coenzyme is bound extremely tightly to the enzyme, it is called a prosthetic group. With their cofactors or coenzymes, enzymes are called holoenzymes, and without cofactors or coenzymes, enzymes are called apoenzymes.
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.