Fundamentals of Organic Chemistry for the MCAT: Everything You Need to Know
/Learn key MCAT concepts about the fundamentals of organic chemistry, plus practice questions and answers
(Note: This guide is part of our MCAT Organic Chemistry series.)
Table of Contents
Part 1: Introduction to the fundamentals of organic chemistry
Part 2: Arrow-pushing mechanisms
a) Double-headed arrows
b) Single-headed arrows
Part 3: Nucleophiles and electrophiles
a) Overview of functional groups
b) Nucleophilic substitution reactions
c) Elimination reactions
Part 4: High-yield terms
Part 5: Passage-based questions and answers
Part 6: Standalone questions and answers
-----
Part 1: Introduction to the fundamentals of organic chemistry
At first blush, organic chemistry can be quite frightening. The topic covers many concepts: from electron movement to functional groups and organic structures. To top it off, many college-level organic chemistry courses require the memorization of complicated, multi-step reactions.
Here is some good news: the organic chemistry content tested on the MCAT may be much less extensive than what you have learned in a college class. In this guide, we will introduce the fundamental concepts of organic chemistry you must be familiar with to succeed on the MCAT. By the end of this guide, you will be well-prepared to tackle more complex organic chemistry topics in our other guides.
Throughout this guide, there are several high-yield terms listed in bold. These terms will also be listed at the end of the guide. Additionally, there are several MCAT-style practice questions for you to test your knowledge of these fundamental principles.
Let’s get started!
-----
Part 2: Arrow-pushing mechanisms
a) Double-headed arrows
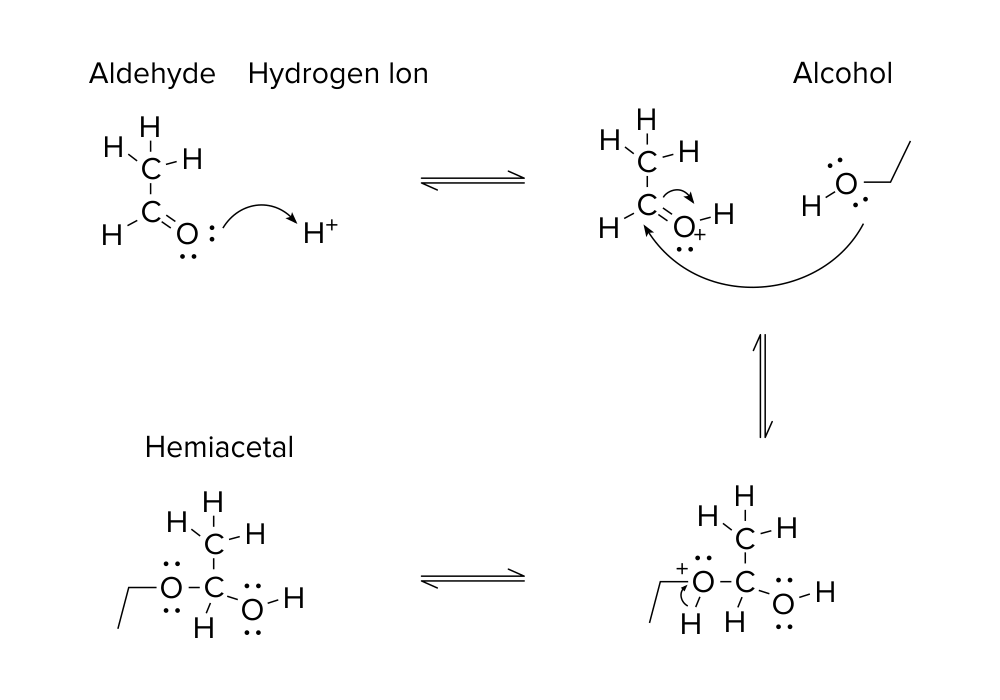
Figure: A hemiacetal-producing reaction
Take a look at the organic reaction above. A quick glance shows that a reaction occurs to produce a new product. A molecule with an aldehyde functional group reacts with a hydrogen ion and alcohol to produce a hemiacetal. (For more information on this topic, be sure to refer to our organic chemistry guide on functional groups.)
All of these molecules are connected by two types of arrows. There are the two half-arrows pointing to the left and to the right in between each step of the reaction. These arrows, known as equilibrium arrows, signify that a reaction is reversible. Thus, each step of the reaction can proceed to the next or previous step with equal probability. Equilibrium arrows are not necessarily always the same length. A reaction can be reversible and have equilibrium arrows of different lengths. The length of the half-arrow corresponds to the likelihood that the organic reaction will travel in that direction.
Mechanisms are illustrated depictions of chemical reactions. Mechanisms are used to illustrate the formation and breaking of various bonds between atoms. In general, mechanisms typically use arrows to depict the movement of electrons. Arrows are generally drawn with an origin at a specific electron pair and point to the bond or atom they attack. Double-headed arrows indicate the movement of an electron pair, or two electrons at once.
Take another look at the illustrated mechanism, and track the movement of electrons between each step.
In the first step, valence electrons from the oxygen atom attack the hydrogen ion to initiate this reaction.
In the second step, there are two double arrows. The first double arrow originates from the valence electrons on the oxygen in the alcohol group and attacks the carbon atom. The second double arrow shows the movement of electrons from the carbon-oxygen double bond to the oxygen atom.
In the third step, electrons from the oxygen-hydrogen bond move to the oxygen, releasing a hydrogen ion.
It’s important to remember that drawing double arrows in a reverse fashion (from a source without electrons) is incorrect and can result in a costly mistake when interpreting a reaction on the MCAT! The arrows extend to the target from the source of electrons.
b) Single-headed arrows
Arrows can be double- or single-headed, with either type having a different meaning.
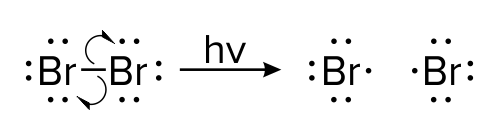
Figure: Light-mediated reaction of a bromine molecule splitting into two ions
In the organic reaction shown above, a single bromine molecule (Br2) splits into two bromine ions (Br-). The catalyst for this reaction is light; in chemical reactions, the letters hv are used to represent high-energy light that breaks bonds. In contrast to the first mechanism, this mechanism uses single-headed arrows.
Single-headed arrows are similar to double arrows in that they show the movement of electrons. Like double-headed arrows, single-headed arrows extend from the source of electrons to the target. In this example, electrons originate in a single bond and are sent to each bromine atom.
Unlike double-headed arrows, single-headed arrows, also referred to as fish hooks, show the movement of only one electron. Thus, as the original bond in the bromine molecule is broken, each resulting ion receives only one electron (instead of a pair of electrons).
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.