Atoms and Periodic Trends for the MCAT: Everything You Need to Know
/Learn essential MCAT chemistry topics on atoms and periodic trends, including atomic radius, ionization energy, electronegativity, and MCAT-style practice questions.
(Note: This guide is part of our MCAT General Chemistry series.)
Table of Contents
Part 1: Introduction to atoms and the periodic table
Part 2: Electronic structure of atoms
a) Atomic structure
b) Electron configuration
c) Electron spin
d) Quantum mechanical model
Part 3: The Periodic Table
a) Groups
b) Periodic trends
Part 4: Atomic phenomena
a) Electron emission
b) Radioactive decay
Part 5: High-yield terms
Part 6: Passage-based questions and answers
Part 7: Standalone questions and answers
-----
Part 1: Introduction to atoms and the periodic table
Atoms are the fundamental unit of matter. As a result, atomic structure and the periodic table form the basis of many general chemistry and organic chemistry MCAT questions.
Several important terms in this guide are bolded. As you work through this guide, we encourage you to create definitions and examples that make the most sense to you! At the end of this guide, there are also MCAT-style practice questions that will test your knowledge on this subject.
Let’s get started!
-----
Part 2: Electronic structure of atoms
Atoms are the smallest unit of matter that can retain a unique identity. This is the result of the structure of individual atoms, which are composed of varying subatomic particles.
a) Atomic structure
Protons, neutrons, and electrons are the subatomic particles that make up atoms. A proton is a subatomic particle that contains a positive charge (+1e). “e” refers to the fundamental unit of charge and one atomic mass unit (AMU). A neutron is a subatomic particle that contains no charge and has a mass of one AMU. Groups of protons and neutrons—collectively referred to as nucleons—form the nucleus of an atom.
An electron is a subatomic particle that contains a negative charge (-1e) and has a mass that is 1823x smaller than that of a proton, or 1/1823 atomic mass units. In other words, when comparing the mass of a proton or neutron to an electron, the mass of an electron is negligible. Electrons can be arranged in shells around an atom. The electrons in the outer shell of an atom are called valence electrons. Valence electrons play an important role in chemical reactions and bonding.
| Subatomic particle | Charge | Mass |
|---|---|---|
0 AMU |
Note that the number of protons determines the element a specific atom is. For example, any atom containing 15 protons will be phosphorus, despite the number of neutrons that the atom has. This number of protons is referred to as the atomic number.
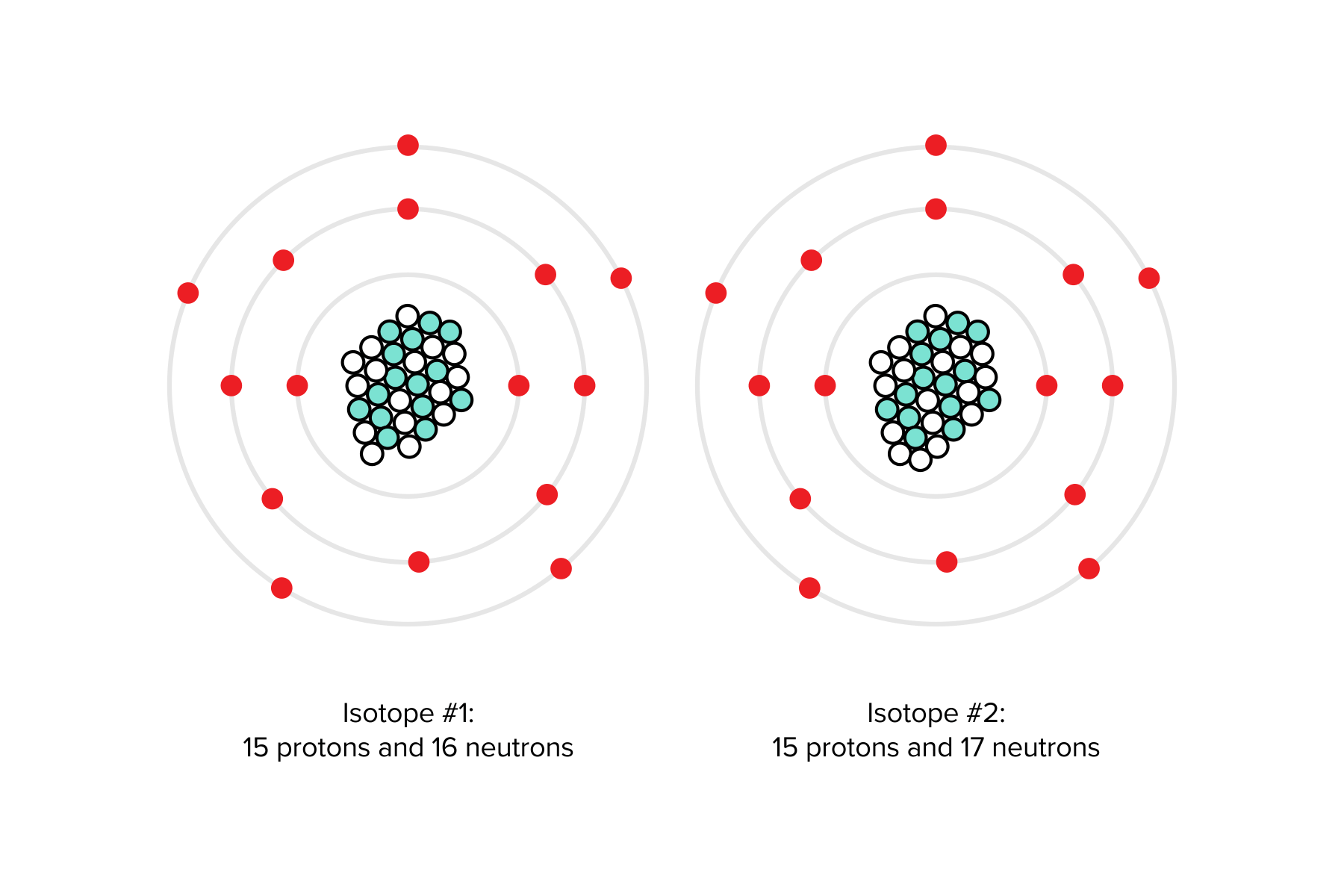
Atoms of the same atomic number but with differing numbers of neutrons are isotopes. Consider this: one isotope of phosphorus might consist of 16 neutrons and 15 protons, while another phosphorus isotope contains 17 neutrons and 15 protons. Both isotopes possess the same atomic number and are considered to be phosphorus but possess differing amounts of neutrons.
Figure: Two possible isotopes of phosphorus.
As a result, two isotopes of an element will possess differing atomic mass: a measure of mass that is calculated by summing the number of protons and neutrons within an atom. The atomic mass of many isotopes is used to calculate atomic weight: a weighted average of masses of all naturally occurring isotopes.
- Atomic number (Z): the number of protons in an atom, which determines the elemental identity of the isotope (e.g., any isotopes with 15 protons must be phosphorus)
- Mass number (A): the number of protons and neutrons in an atom (e.g., 30P contains 15 protons and 15 neutrons, resulting in an atomic mass of 30)
- Atomic weight: the weighted average of the masses of naturally occurring isotopes (e.g., if half of all naturally occurring isotopes are 30P and the remaining half is 31P, the atomic weight would be the average of the two mass numbers multiplied by their abundance. Since both isotopes are equally abundant, the atomic weight is 30.5 AMU.)
How is it possible that so many protons with positive charges are held together so tightly in the nucleus? When considering that like charges repel, shouldn’t the positive charges of the protons cause the nucleus to rip apart?
The strong nuclear force is a fundamental force that prevents this. In particular, it is an attractive force that holds protons and neutrons together—despite any alike positive charge.
b) Electron configuration
How are subatomic particles arranged to form an atom? While protons and neutrons are held together in the nucleus by the strong nuclear force, the arrangement of electrons outside the nucleus is much more complex.
An electron configuration is written by determining the number of electrons that are present in the species at hand. For most atoms, or neutrally charged particles, this number of electrons is equal to the atomic number of the element. (Why is this? Recall that the charge of one electron is equal to the charge of one proton. Thus, if a particle has zero charge, the number of electrons and number of protons must be equal). For ions, or particles with a net positive or negative charge, the number of electrons may not be equal to the atomic number of the element.
When “filling” in an atom, electrons are placed in concentric shells surrounding an atom. While these shells are not physical structures, they are useful in visualizing the spaces that electrons may occupy.
The number of concentric shells is determined by the group number of an atom. Thus, elements in the first row of the periodic table have one electron shell, elements in the second row have two electron shells, and so forth. As a general rule, electron shells of a higher number are located further from the nucleus. The outermost electron shell, or highest-numbered electron shell, is located the furthest. This outermost shell is also called the valence shell.
Valence electrons are electrons on the outermost shell of an atom. These electrons are responsible for the reactivity and individual properties of atoms. Each atom is at its most energetically stable when its valence shell is full of electrons. Some elements, like noble gases, already have a full shell.
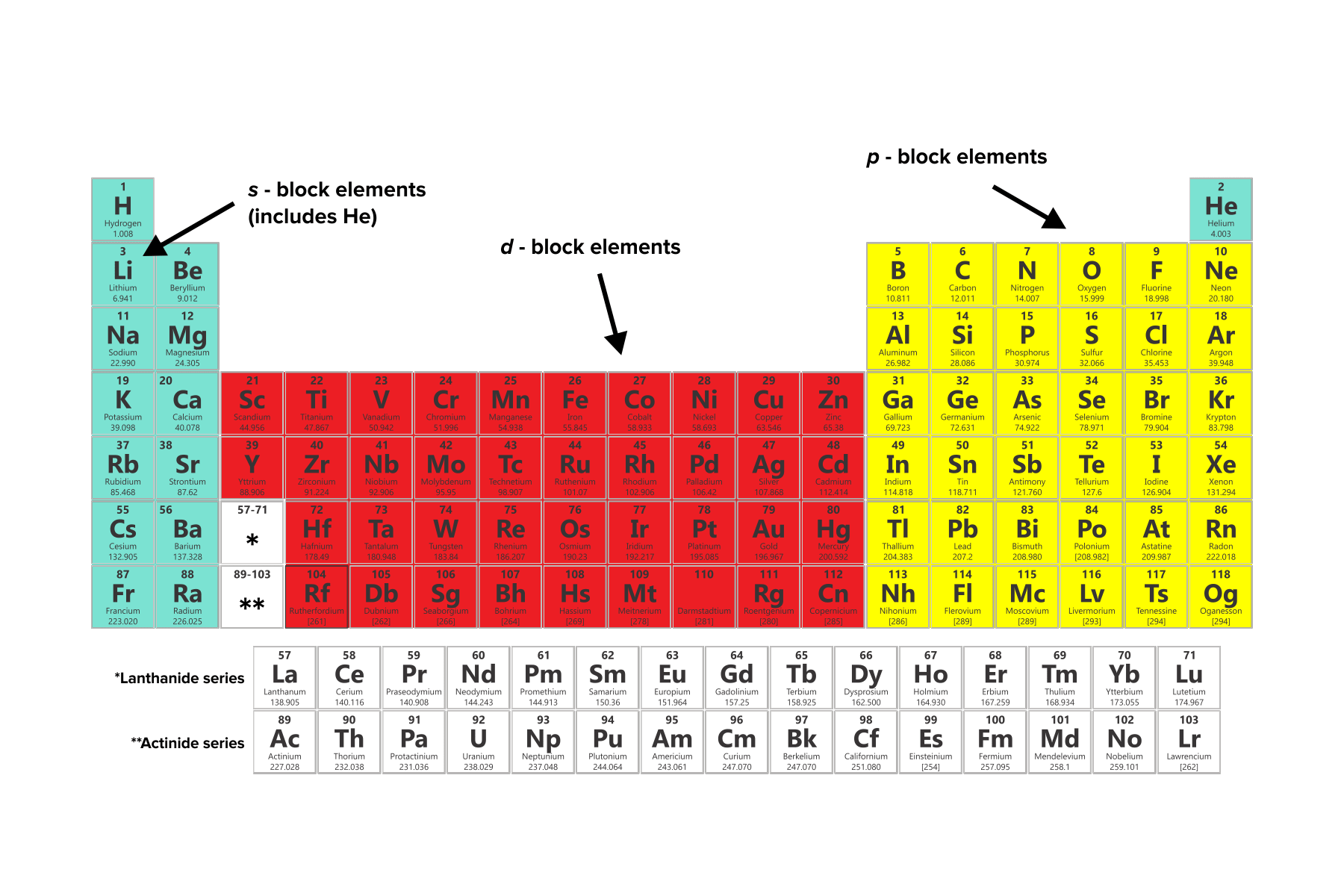
Each shell is composed of orbitals: regions of certain shapes in which electrons are likely to be found. While there are many orbital shapes that are possible, the MCAT will focus on three particular orbitals denoted as s, p, and d. Elements can be categorized into s-block, p-block, and d-block elements based on their highest-energy orbital.
Figure: Elements categorized by outermost valence electron orbitals.
Categorizing elements based on their highest-energy orbital loosely correlates with the type of element each is. Most s-block elements and f-block elements are metals. The p-block elements are a mixture of metalloids and nonmetals.
There are two important considerations in determining the electron configuration of a neutral atom:
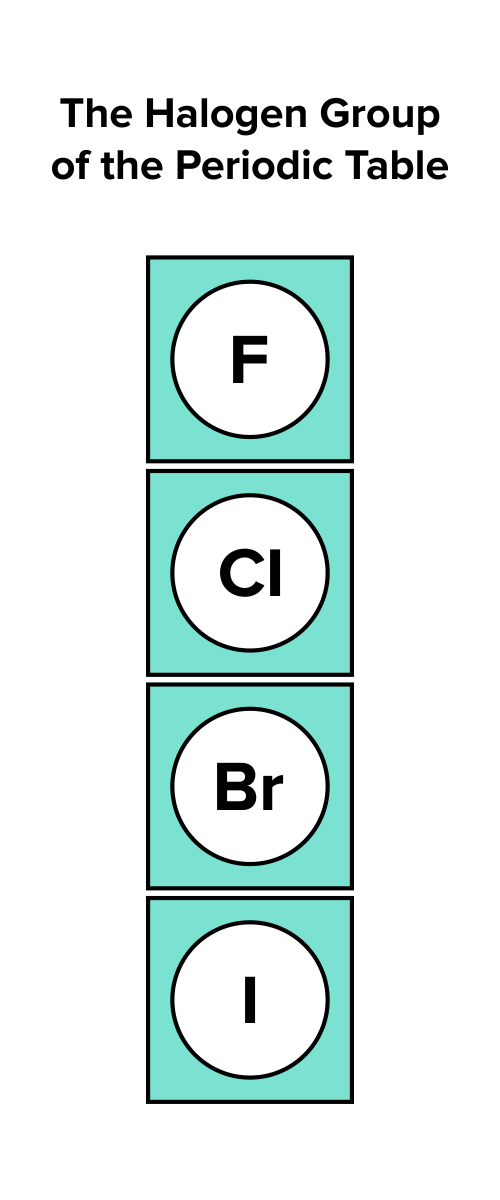
Determine the group number of the atom. Determine the group number of the atom by identifying the column of the periodic table the atom is present in. For instance, the halogens are in the seventeenth group, or column, or the periodic table.
Figure: Elements in the seventh group.
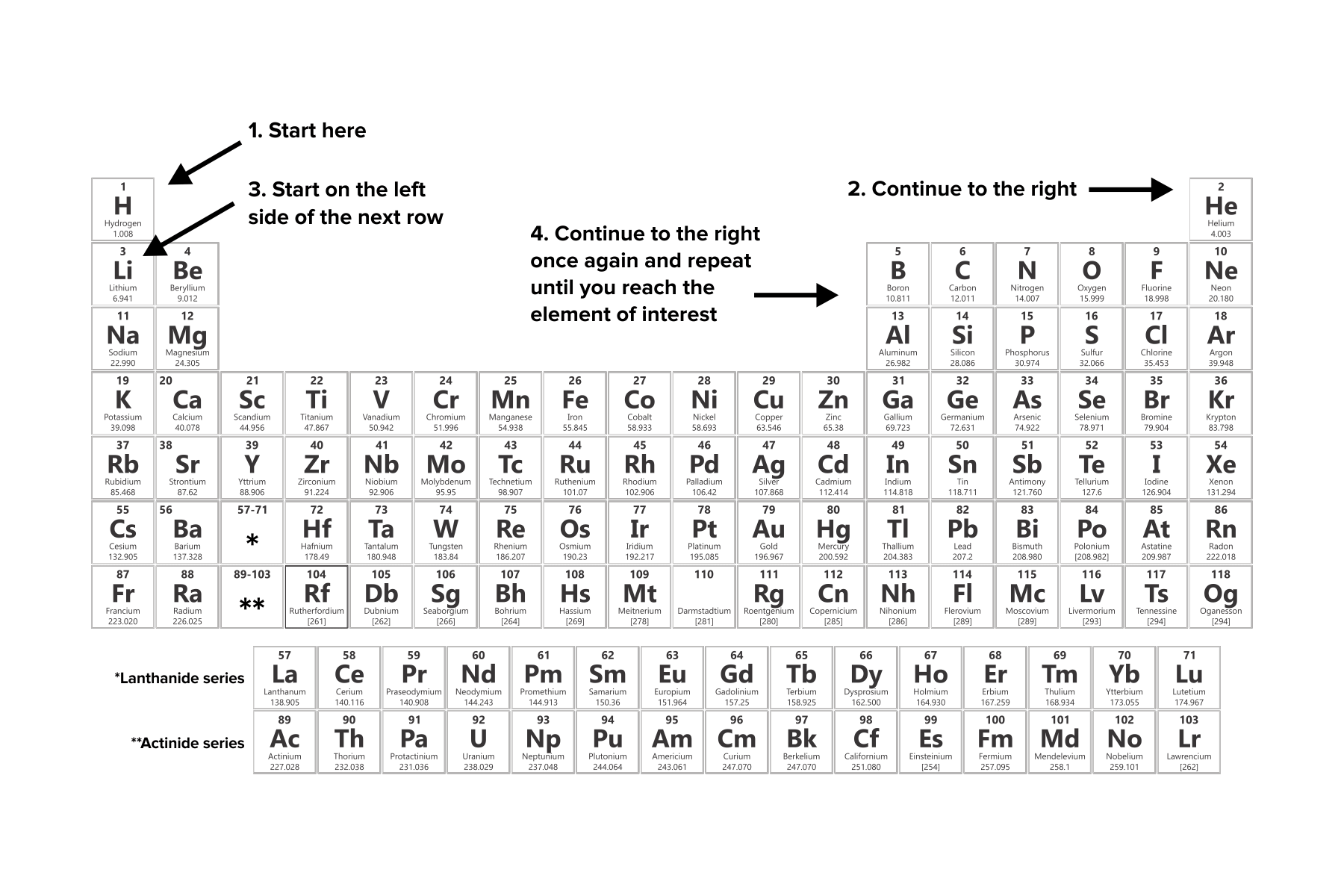
2. Understand the path of filling electrons in shells. Electrons are filled in shells based on a predetermined order.
All filling begins in the first “subshell,” or the first orbital (e.g., that of hydrogen).
Filling continues to the right of the periodic table. When one row, or family, is completed, filling continues with the leftmost element of the succeeding row.
The Aufbau principle states that each orbital in a shell must be sequentially filled before electrons can begin to fill the next shell.
Filling is concluded when the appropriate number of electrons is placed into orbitals. For neutrally charged atoms, this number of electrons is equal to the atomic number of the element.
Figure: Guidelines for filling electron configurations.
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.