Carbohydrates for the MCAT: Everything You Need to Know
/Learn key MCAT concepts about carbohydrates, plus practice questions and answers
(Note: This guide is part of our MCAT Biochemistry series.)
Part 1: Introduction
Part 2: Useful reactions
a) Fischer and Haworth projections
b) Cyclization
Part 3: Sugar structures
a) High-yield monosaccharides
b) High-yield disaccharides
c) Glycogen
Part 4: High-yield terms
Part 5: Passage-based questions and answers
Part 6: Standalone questions and answers
----
Part 1: Introduction
Although modern humans have been around for at least 100,000 years, one topic that seems to puzzle us to this day is our diet. In grocery stores all across the United States, you’ll find plenty of food products marketed as “low-carb” or “keto-friendly.” You may have even heard of famous athletes, such as Lebron James and Tim Tebow, sharing their results on a low-carb diet.
Despite the bad press, carbohydrates certainly played—and continue to play—a significant role in our evolutionary history and shaped who we are today. Thus, it’s no surprise that you are expected to understand them in detail as you pursue a career in medicine. In this article, we’ll discuss the basic biochemistry behind carbohydrates so that you can have a strong foundation to build on when reviewing their metabolism.
Without further ado, let’s begin!
----
Part 2: Useful reactions
a) Fischer and Haworth projections
Carbohydrates, also commonly referred to as “sugars,” can be represented in many forms. One such form is a Fischer projection, a two-dimensional representation of a molecule that gives three-dimensional information. The horizontal lines of a Fischer projection are equivalent to wedges: functional groups on horizontal lines are coming out of the page. The vertical lines are equivalent to dashes: functional groups on vertical lines are going into the page.
Figure: Fischer projections of D- and L- forms of glyceraldehyde.
For sugars, the absolute configuration is designated using D- and L- nomenclature instead of the R and S system used in organic chemistry. If the hydroxyl group of the highest-numbered chiral carbon is on the right, the sugar is in the D-configuration. If the hydroxyl group of the highest-numbered chiral carbon is on the left, it is in the L-configuration.
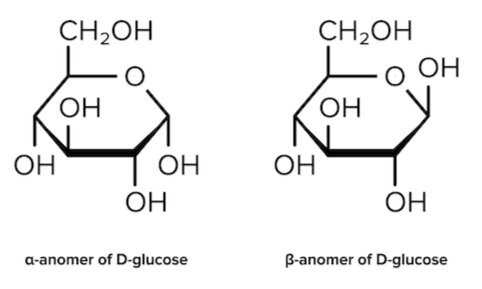
While Fischer projections represent the straight-chain form of carbohydrates, you may also see sugars represented in their cyclic form as Haworth projections. In a cyclic sugar, the anomeric carbon is the carbon that has two bonds to oxygen.
Be aware that a cyclic sugar can exist in two possible anomers: an ⍺-anomer and a β-anomer. A sugar is in its α-anomer form when the anomeric carbon’s substituent is on the opposite side of the plane as the highest numbered chiral center’s substituent. A sugar is in its β-anomer form when the anomeric carbon’s substituent is on the same side of the plane as the highest numbered chiral center’s substituent.
Figure: Haworth projections of the α- and β-anomers of D-glucose.
b) Cyclization
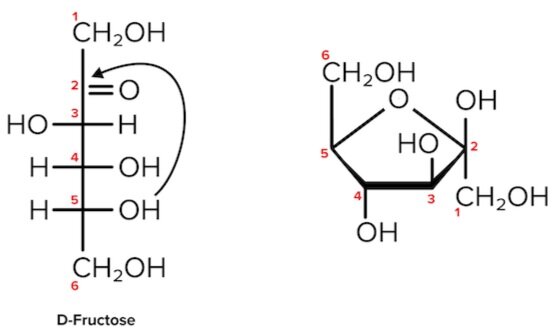
Cyclic sugars are formed via an intramolecular reaction, or a reaction between two functional groups on the same molecule. Aldoses are sugars with an aldehyde group (-CHO). Their cyclization forms a hemiacetal. On the other hand, Ketoses have a ketone group (-CO) and no other further oxidized functional groups. Their cyclization forms a hemiketal.
It’s easiest to visualize this reaction when the carbohydrate is drawn in its linear form (e.g., in a Fischer projection). During cyclization of both aldoses and ketoses, the hydroxyl group (nucleophile) on the highest-numbered chiral center attacks the carbonyl group (electrophile).
Figure: Cyclization of D-fructose, a hemiketal. The second carbon is both the site of nucleophilic attack and the anomeric carbon (as it is bonded to 2 oxygen atoms).
Note that as we convert the structure of the sugar from its two-dimensional to three-dimensional structure, the functional group that is pointing to the right of the Fischer projection will end up pointing downward in the Haworth projection.
For most sugars that are dissolved in a solution, this cyclization occurs spontaneously. As a result, many sugars can be found in various combinations of D-, L-, and linear forms. (The final ratios of each of these forms are usually affected by stereochemistry unique to each molecule.) This tendency to undergo spontaneous cyclization is known as mutarotation.
Sugars can also be described as being “non-reducing” or “reducing.” A reducing sugar is one that can act as a reducing agent. Reducing sugars can be identified through the presence of a free anomeric carbon, meaning it is not in a glycosidic bond and has a free hydroxyl group. Conversely, non-reducing sugars lack a free anomeric carbon.
Benedict’s reagent and Tollen’s reagent are two reagents that are commonly used to detect the presence of reducing sugars. The two reagents react with reducing functional groups in unique ways: Benedict’s reagent reacts with aldoses to form a red copper precipitate, while Tollen’s reagent reacts with aldehydes to form a silver, mirrorlike precipitate.
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.