Bonds and Interactions for the MCAT: Everything You Need to Know
/A complete guide to MCAT bonds and interactions, covering key concepts, test strategies, and practice questions to improve your chemistry score.
(Note: This guide is part of our MCAT General Chemistry series.)
Table of Contents
Part 1: Introduction to bonds and interactions
Part 2: Interatomic forces
a) Ionic bonds
b) Covalent bonds
c) Coordinate covalent bonds
d) Single and double bonds
e) VSEPR theory
Part 3: Intermolecular forces
a) Hydrogen bonds
b) Dipole-dipole interactions
c) London dispersion forces
Part 4: High-yield terms
Part 5: Passage-based questions and answers
Part 6: Standalone questions and answers
-----
Part 1: Introduction to bonds and interactions
What holds everything in the universe together? Why don’t atoms and molecules randomly split apart and float away? The answer lies in bonding and molecular interactions. Understanding how atoms and molecules interact with each other is crucial for much of the chemistry section of the MCAT. Atomic bonds and intermolecular attractions are the foundation for many additional concepts in organic chemistry and biochemistry.
Chemical bonds are chemical interactions that create an attractive force between atoms or molecules. There are a variety of bonds, or attractive forces, that exist. Some exist between individual atoms of a molecule, while others exist between molecules.
Throughout this guide, there will be several important terms marked in bold. Be sure to understand these terms well, as they will be high-yield on Test Day! At the end of this guide, there are also several MCAT-style questions you can use to test your knowledge.
Let’s get started!
-----
Part 2: Interatomic forces
a) Ionic bonds
The simplest form of interatomic bond is known as an ionic bond. Ionic bonds are formed through the interaction of two atoms with vastly different electronegativities. Because both atoms have different electronegativities, one atom donates electrons to another. This results in the creation of a positively charged ion (cation) and a negatively charged ion (anion). The negatively and positively charged atoms attract, creating an ionic bond.
Because ionic bonds take advantage of different electronegativities, the constituent elements usually have quite different characteristics. In fact, ionic bonds are typically composed of metal and nonmetal. Sodium chloride (chemical formula: NaCl) is a molecule that is held together by an ionic bond. Sodium is a highly reactive metal, and chlorine is a highly reactive gas. Together, they form table salt, which we consume every day!
Figure: A sodium atom and a chloride ion form an ionic bond to create table salt.
b) Covalent bonds
The second form of interatomic bond is the covalent bond. A covalent bond is usually formed between two atoms with similar electronegativities. Thus, covalent bonds are usually formed between nonmetals or metalloids.
Because the atoms have similar electronegativities, the atoms do not donate electrons to each other. Instead, they share electrons. This sharing between the two atoms can either be equal or unequal. Whether atoms in a covalent bond equally or unequally shared electrons depends on their difference in electronegativity. The greater the difference in electronegativity between two elements is, the more partial ionic character (e.g., the more similarity to an ionic bond) the bond between them has.
The polarity of a covalent bond can be described through a dipole moment. The dipole moment is a quantity that can be calculated using the following equation:
p=qd
where p = dipole moment,
q = net charge,
d = distance between the partial charges
This equation may look similar to another law: Coulomb’s law, which describes the attractive force generated between two charged particles. Here, the dipole moment describes an analogous quantity. A large dipole moment indicates a larger polarity, or difference in electron distribution, between partial charges.
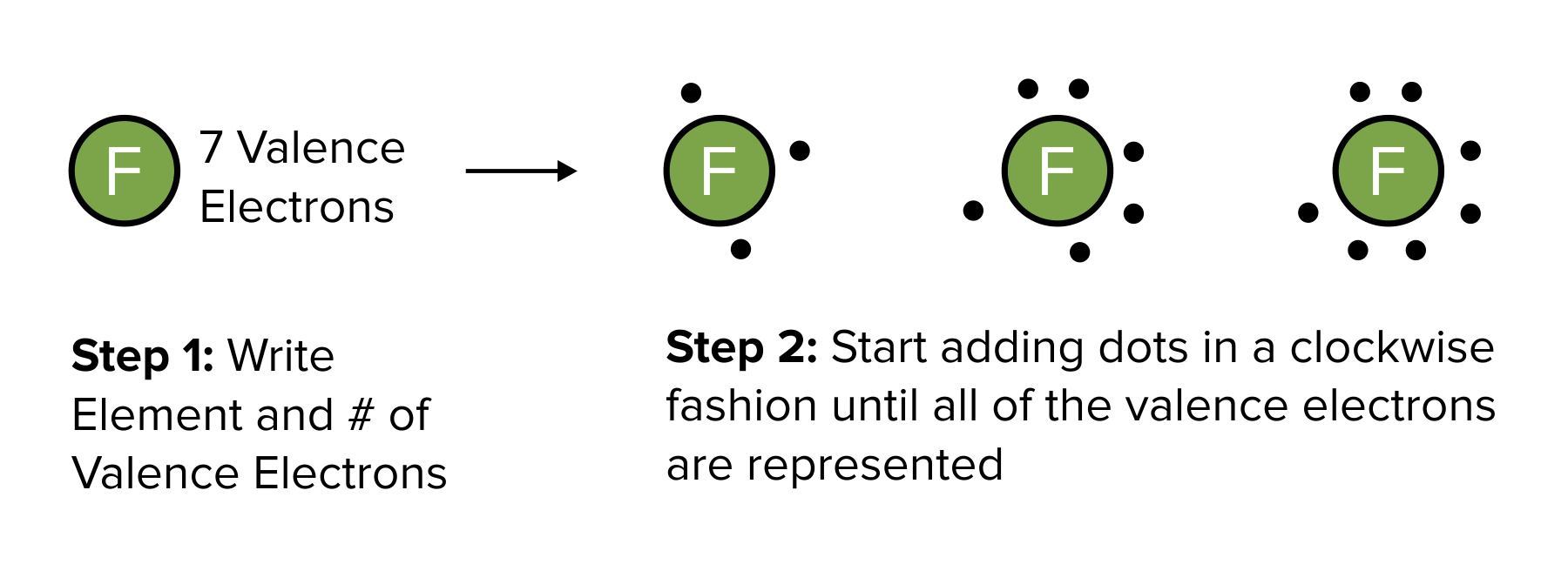
Lewis structures are a common method of representing covalent bonds. In Lewis dot structures, electrons in the valence orbitals are represented by a dot on each side of an atom’s elemental symbol. (To learn more about valence electrons, be sure to refer to our guide on atoms and periodic trends.)
To draw a Lewis dot structure, begin by writing the elemental symbol for the atom of interest. Then, calculate the number of valence electrons in the s and p orbitals. Once calculated, place one dot to the top of the elemental symbol. Continue placing dots in a clockwise manner around the symbol until the number of dots equals the number of valence electrons. This process is repeated for each element in a covalent bond.
After each atom participating in a covalent bond is illustrated, electrons participating in sigma bonds can be connected with a line.
Figure: Constructing a Lewis structure.
Figure: Comparing two resonance structures
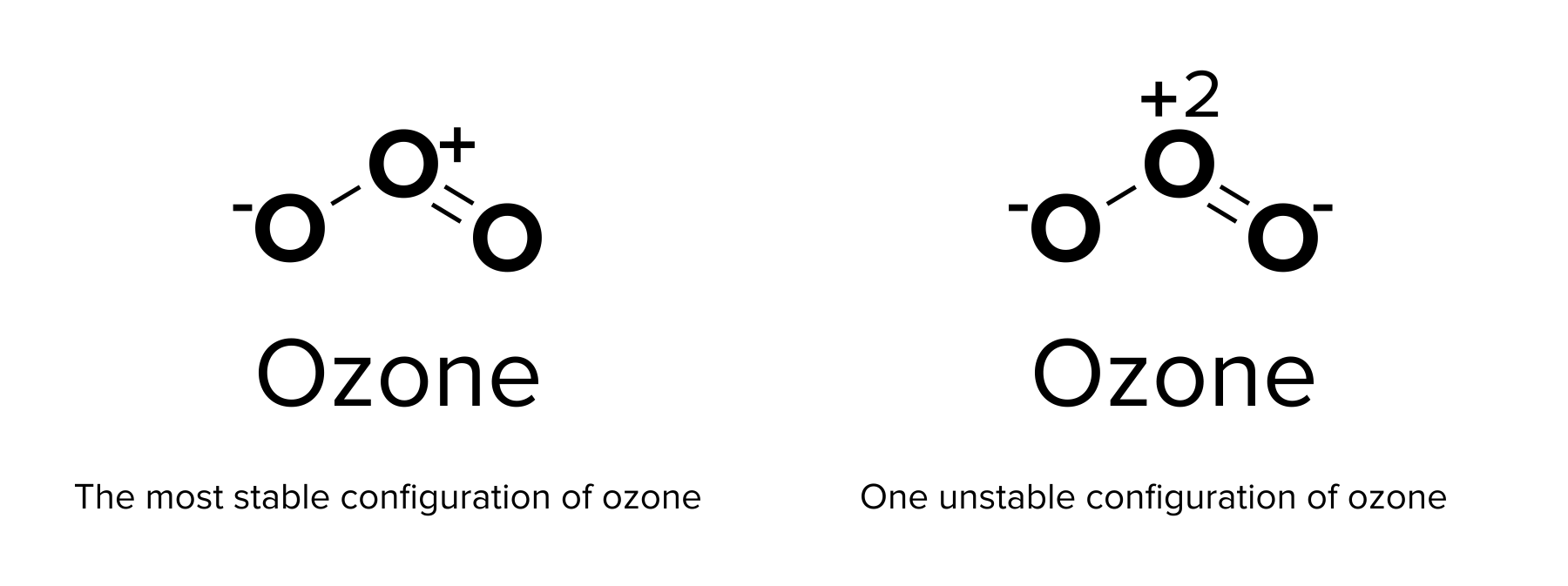
One advantage to using a Lewis dot diagram is that resonance structures can be shown. A resonance structure is an alternative model of covalent bonding. A molecule that displays resonance may have electrons in any of these resonance configurations at any given time. In general, the bonds within such a molecule are assumed to be a combination of all of these resonance structures. The electrons within the molecule are said to be delocalized and can be found with equal probability within any of these resonance structures. The resulting combined configuration may be shown with a dashed line.
The most stable resonance form is one that provides the lowest formal charge of the atoms. Formal charge refers to the amount of “functional” charge an atom would have, assuming all electrons are shared equally. Thus, it can be calculated using the following equation:
formal charge = # valence electrons - nonbonding electrons - bonding electrons ÷ 2
Recall our previous discussion of ozone. In the ozone molecule, each oxygen atom has a different formal charge. One atom has a charge of 0, while the other two have charges of -1 and +1. This is the relatively stable form of ozone, as the formal charges of each species are fairly low. In contrast, creating only single bonds in ozone would create formal charges of -1, -1, and +2, which are much higher formal charges. This resonance form is highly unlikely to occur!
Molecules and chemical compounds can often be drawn in a variety of ways. For more information on this, be sure to refer to our guide on isomers.
c) Coordinate covalent bonds
Recall that covalent bonds are simply bonds formed between atoms in which electrons are unevenly shared. A coordinate covalent bond, sometimes referred to as a dative bond, is a special subtype of covalent bond that often forms between transition metals. In this type of covalent bond, both electrons in the bond are donated by a single atom.
Due to their unique orbital structure, transition metals can easily accept electrons from other atoms. For this reason, transition metals can form two, three, or four coordinate covalent bonds with other atoms! Zinc, iron, and magnesium are examples of such metals that usually bond with enzymes inside our bodies. Since they are integrated into enzymes and are often critical for the enzyme’s function, these transition metals are referred to as cofactors. (For more information on enzymes and cofactors, be sure to refer to our guide on enzymes.)
d) Single and double bonds
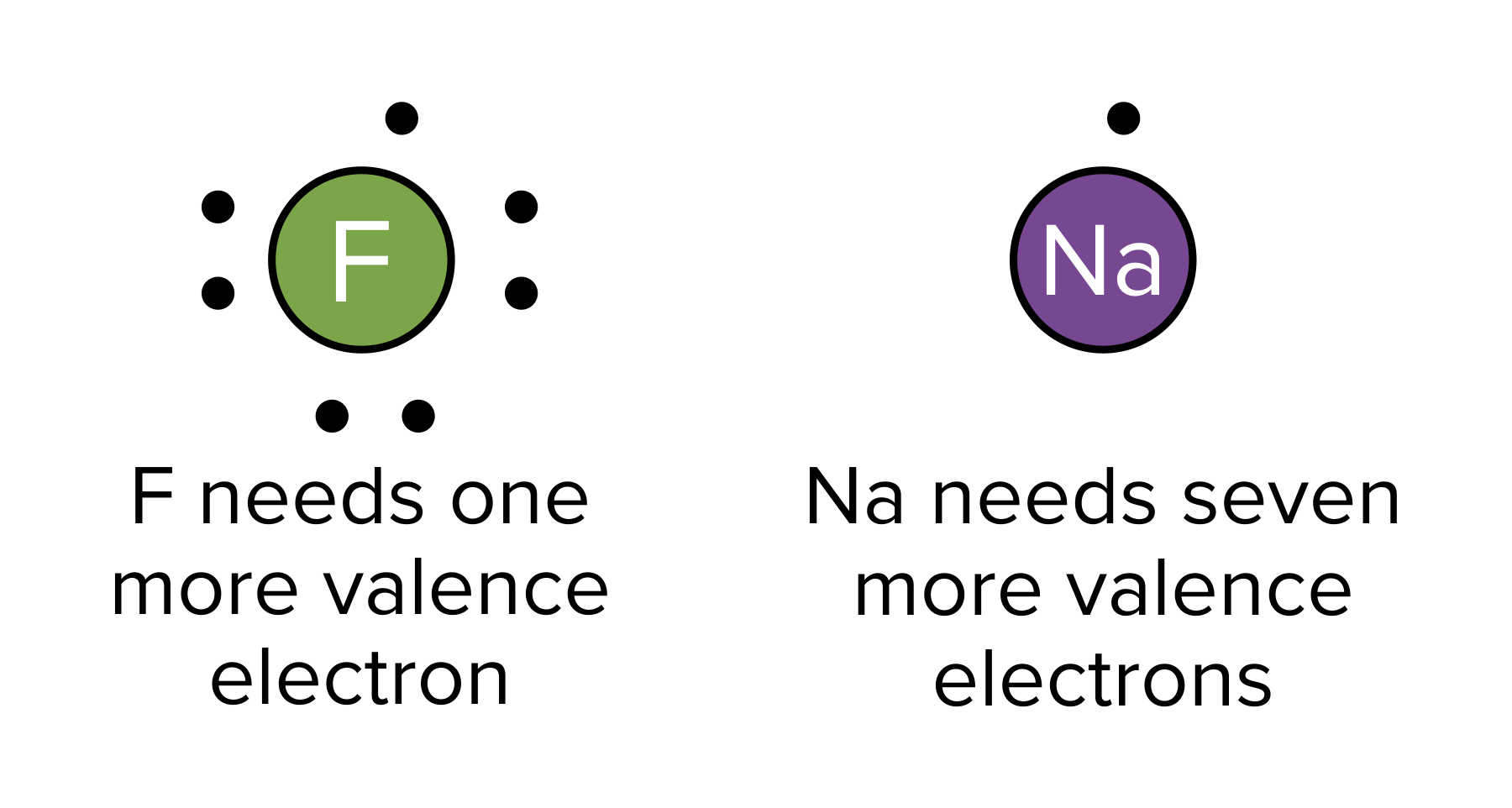
In chemistry, the octet rule states that each atom is most stable when it contains a full valence shell of 8 electrons. This means that atoms with 1 electron in their valence shell are ready to give up their electrons while those with a nearly full shell aren’t. Thus, each atom in a molecule must be exposed to eight electrons: either through electron transfer (e.g. ionic bonding) or sharing (e.g., covalent bonding).
Figure: Atoms seek to obtain a full octet of valence electrons.
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.