Stereochemistry for the DAT
/Learn key DAT concepts about stereochemistry, plus practice questions and answers
Learn everything you need to know regarding stereochemistry for the dat
Table of Contents
Part 1: Introduction to stereochemistry
Part 2: Structural isomers
Part 3: Chirality
a) Chiral centers
b) R, S configuration
c) E, Z configuration
d) Diastereomers and enantiomers
e) Conformational isomers
Part 4: In biological systems
a) Amino acids
b) Carbohydrates
c) Boat and chair conformations
Part 5: Sterics and stability
a) Sterics
Part 6: High-yield terms
Part 7: Questions and answers
----
Part 1: Introduction to stereochemistry
Stereochemistry describes how atoms are arranged in 3-dimensional molecules. As you will learn in this guide, stereochemistry and isomers have a big impact on everyday life. For example, your body only produces L amino acids and not D amino acids. Stereochemistry is also important in drug manufacturing, as one enantiomer of a drug can be more effective than the other.
As you study this topic, pay attention to any bolded terms, especially terms that classify isomers such as enantiomer or diastereomer. The DAT likes to ask questions that require you to understand these terms, so be sure to spend enough time learning them.
----
Part 2: Structural isomers
Remember that covalent bonds are established through the sharing of one or more pairs of electrons between atoms. These covalent bonds not only orient atoms in space but also exert a significant influence on the overall molecular geometry. To explore this concept in greater detail, you can refer to our comprehensive guide on atomic and molecular structure.
Consequently, covalent bonds play a pivotal role in relation to stereochemistry and isomers. Stereochemistry is the 3-D arrangement of atoms and bonds in a molecule. It is imperative to always consider chemical structures within their three-dimensional configurations. Isomers are two or more molecules that have the same molecular formula, but differ in 3-D shape or connectivity of atoms. Structural isomers are a type of isomer that exhibit distinct three-dimensional structures due to the unique ways atoms are arranged through covalent bonds.
To illustrate this, let's compare two molecules, both with the formula C4H10: butane and isobutane. While both butane and isobutane share four carbon atoms and ten hydrogen atoms, the specific arrangement of atoms within the molecules differs.
FIGURE 1: REPRESENTATIONS OF STRUCTURAL ISOMERS, BUTANE AND ISOBUTANE, SHARING THE SAME CHEMICAL FORMULA YET EXHIBITING DISTINCT ATOMIC CONNECTIONS.
The orientation of atoms in three-dimensional space is crucial when discussing isomers, and depicting these arrangements on a two-dimensional surface can be challenging. As per convention, bonds represented by wedges are considered to project "out of" the page, while bonds represented by dashes are regarded as receding "into" the page. Bonds drawn as solid lines are situated within the same plane as the page.
FIGURE 2: THE STRUCTURAL REPRESENTATION OF CYSTEINE UTILIZING DASHES AND WEDGES.
----
Part 3: Chirality
Take a moment to observe your left and right hands. Position them with both palms facing upward and overlap your left hand onto your right hand. You'll notice that your hands cannot be perfectly matched; they are non-superimposable. Instead of aligning perfectly, the pinky of your left hand corresponds to the thumb of your right hand.
Your hands exhibit a characteristic similar to chiral molecules. Chiral molecules are essentially mirror images of each other but cannot be superimposed. The concept of chirality is closely tied to symmetry. Think of how a molecule can be divided into symmetrical halves by a plane of symmetry. For instance, boron trifluoride (BF3) possesses a plane of symmetry that neatly splits the molecule into two identical halves that reflect each other.
FIGURE 3: BORON TRIFLUORIDE FEATURES AN INTERNAL PLANE OF SYMMETRY THAT RESULTS IN TWO IDENTICAL HALVES.
In contrast, chiral molecules lack a plane of symmetry. Take lactic acid as an example; it lacks a plane of symmetry that could bisect the molecule into mirror-image halves. Consequently, the mirror images of lactic acid cannot be superimposed onto each other, making lactic acid a chiral molecule.
FIGURE 4: AN ILLUSTRATION OF A CHIRAL MOLECULE, LACTIC ACID.
The upper part of the figure shows the molecule reflected upon itself, while the lower part illustrates the mirror images of lactic acid, which cannot be superimposed onto each other.
a) Chiral centers
A chiral center is an atom with four distinct, different substituents attached to it. In most cases, a molecule containing at least one chiral center is considered chiral, while a molecule lacking chiral centers is termed achiral. Achiral molecules are super imposable.
Chiral centers are frequently sp3-hybridized carbon atoms. Sp3 hybridization indicates that the atom is bonded to four different groups, employing one s orbital and three p orbitals. This results in a tetrahedral molecular shape.
You may see DAT questions that ask you to identify the number of chiral centers in a molecule. It can be useful to recognize which atoms are sp3-hybridized when searching for potential chiral centers within a given molecular structure.
It's important to note that an sp3-hybridized carbon may not be a chiral center, especially if some of its four substituents are the same. Take methanol (CH4O), for instance. Despite the central carbon being sp3-hybridized, it is not connected to four distinct substituents, and as a result, the molecule can be superimposed onto its mirror image.
FIGURE 5: THE STRUCTURAL REPRESENTATION OF METHANOL.
Chiral molecules often exhibit optical activity, which means they can interact with and polarize light. However, a compound with multiple chiral centers may not necessarily display optical activity.
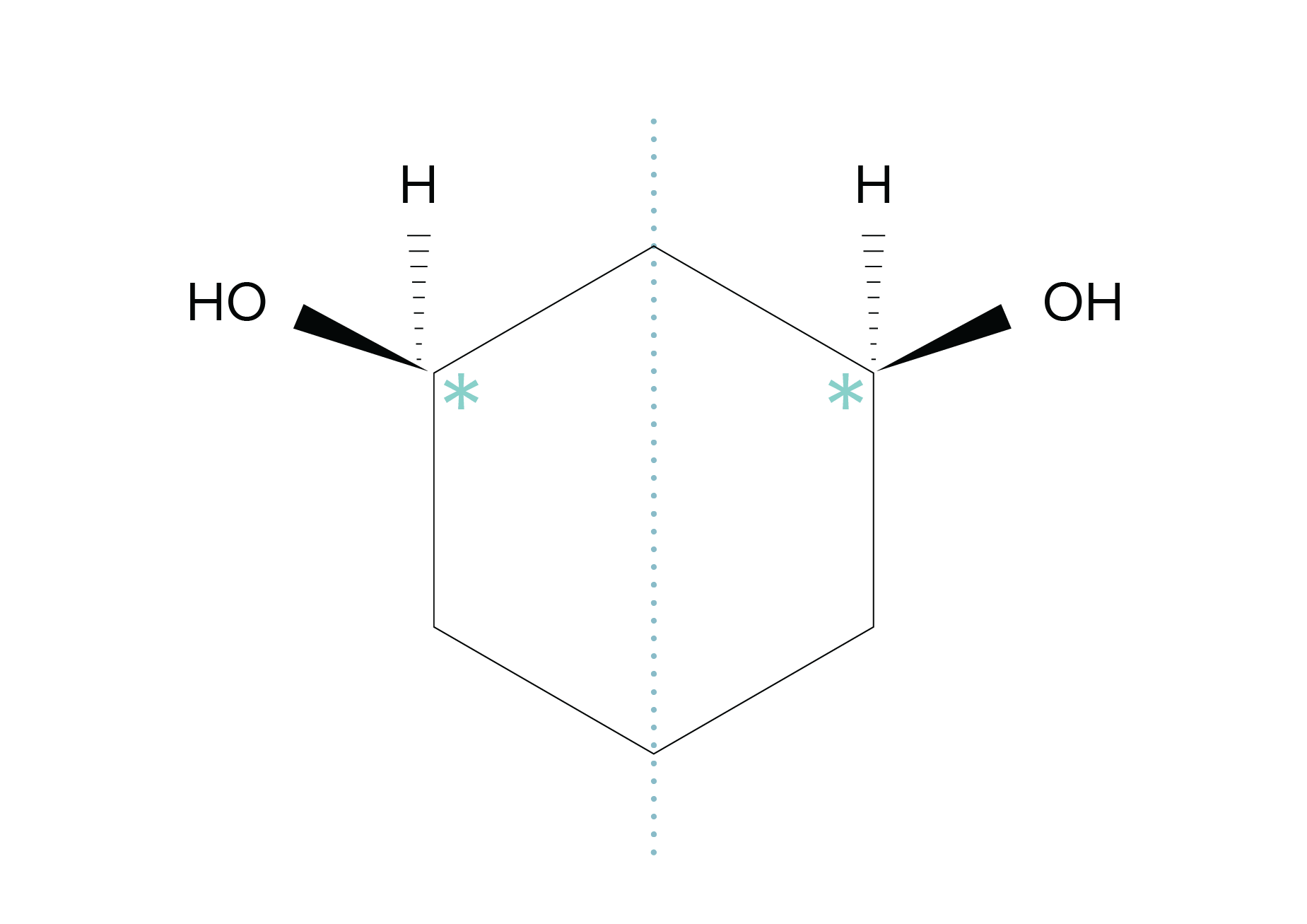
In certain cases, a molecule may possess multiple chiral centers but remain achiral. Meso compounds are a prime example of this scenario. Meso compounds contain multiple chiral centers and also possess an internal plane of symmetry. Consequently, a meso compound with multiple chiral centers can still be considered achiral overall. To determine if a compound is meso, first confirm that it contains at least two chiral centers. Then, attempt to divide the structure into two halves through a plane of symmetry. If the compound exhibits the same arrangement of substituents on both sides, it is classified as meso.
FIGURE 6: AN ILLUSTRATION OF A MESO COMPOUND WITH TWO CHIRAL CENTERS (INDICATED WITH * SYMBOLS) AND AN INTERNAL PLANE OF SYMMETRY. THIS STRUCTURE IS NOT CHIRAL, DESPITE THE PRESENCE OF TWO CHIRAL CENTERS.
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.