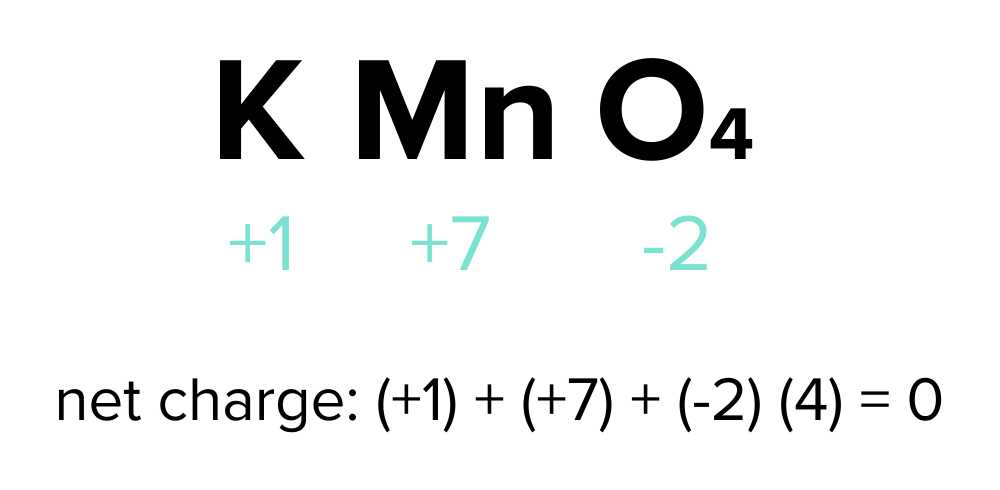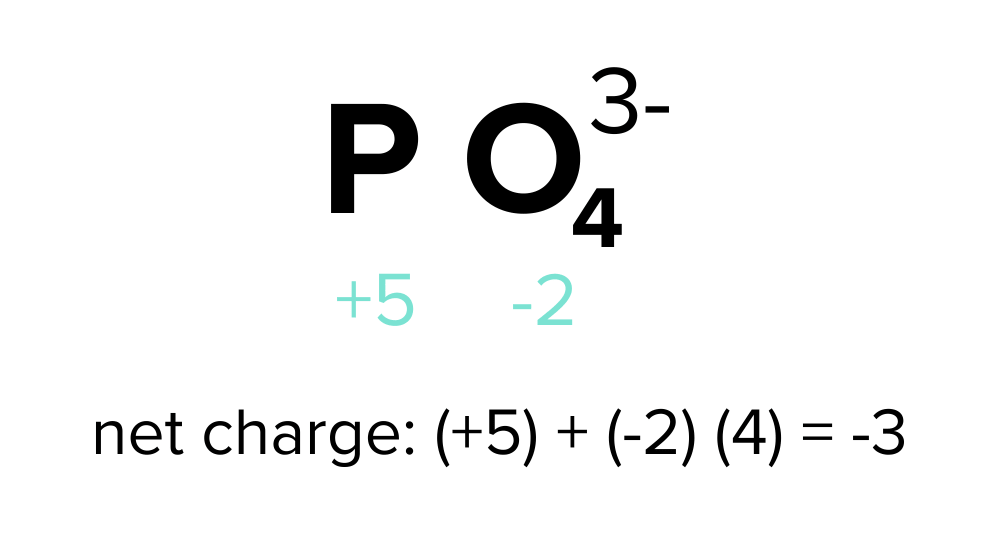Oxidation-Reduction Reactions for the DAT
/Learn key DAT concepts about oxidation and reduction reactions, plus practice questions and answers
Everything you need to know about oxidation-Reduction for the dat
Table of Contents
Part 1: Introduction to oxidation-reduction reactions
Part 2: Oxidation and reduction
a) Definitions
b) Assigning oxidation states
c) Hydrides
Part 3: Applications of redox reactions
a) Writing and balancing redox reactions
b) Electron transport chain
Part 4: Cell potentials
a) Oxidation and reduction potentials
b) Electromotive force
Part 5: Concentration cells
a) Calculation of electric potential
b) Nernst equation
Part 6: Electrochemical cells
a) Voltaic cells
b) Electrolytic cells
c) Redox reactions in electrochemical cells
d) Batteries
Part 7: High-yield terms
Part 8: Questions and answers
----
Part 1: Introduction to oxidation-reduction reactions
Oxidation and reduction, collectively known as redox reactions, constitute fundamental concepts in general chemistry. These reactions dynamically exchange electrons during chemical transformations. In general chemistry, understanding redox reactions is pivotal, as they are a part of various chemical processes such as the corrosion of metals or production of energy in batteries. This guide will teach you everything you need to know about redox reactions, as well as electrochemical cells for the DAT. As you study these topics, pay attention to the high-yield bold terms and test yourself with DAT-style questions and answers.
----
Part 2: Oxidation and reduction
a) Definitions
You might be familiar with the concept that oxidation involves a loss of electrons, while reduction involves a gain of electrons. An effective mnemonic for this is "LEO says GER," signifying "Losing Electrons is Oxidation, and Gaining Electrons is Reduction." Alternatively, the mnemonic "OIL RIG" conveys a similar idea: "Oxidation Is Loss, Reduction Is Gain." The term redox encapsulates both processes, combining "reduction" and "oxidation."
Consider neutral copper (Cu) as an example, which becomes oxidized when it loses or donates electrons to become a positive ion (Cu2+). Conversely, a compound undergoes reduction when it gains electrons, such as elemental oxygen (O2) transforming into water (H2O).
It's crucial to differentiate between an oxidizing agent and a compound that undergoes oxidation. The terms "oxidizing" and "reducing" describe the impact these agents or compounds have on another substance; they do not imply that the compound itself is being oxidized or reduced. Consequently, an oxidizing agent (or oxidant) is a compound that undergoes reduction while facilitating the oxidation of a different compound. Conversely, a reducing agent (or reductant) is a compound that undergoes oxidation, aiding in the reduction of another compound by donating electrons.
Due to the electron exchange nature, oxidation and reduction reactions are inherently interconnected. This means that as one element undergoes oxidation (loses electrons), another element must undergo reduction (receives those electrons).
An exception to this rule is found in disproportionation reactions, where one element can experience both oxidation and reduction simultaneously. In such cases, atoms of the same element act as both the oxidizing agent and the reducing agent.
b) Assigning oxidation states
An atom's oxidation state denotes the extent of its oxidation, signifying the degree of electron loss. Hence, another way to define oxidation and reduction involves considering oxidation states. When an atom undergoes oxidation, its oxidation state rises, and when reduced, it decreases.
Oxidation states may assume negative, zero, or positive values. In the oxidized state, denoting electron loss, the oxidation number becomes more positive compared to the initial oxidation state. For instance, as elemental copper (Cu) oxidizes to form Cu2+, its oxidation state transitions from 0 to +2. Conversely, in the reduced state signifying electron gain, the oxidation number becomes more negative. For instance, elemental oxygen (O2) undergoing reduction to form water experiences an oxidation state shift from 0 to -2.
Assigning oxidation states follows specific rules, with exceptions being uncommon in typical study scenarios. Although the rules may seem extensive, they often align with periodic table trends, making them easier to learn.
Key rules include:
- The oxidation state of uncharged, free elements is consistently 0. This includes all naturally occurring diatomic elements.
- Alkali metals (Group 1) have an oxidation state of +1.
- Alkaline earth metals (Group 2) consistently exhibit an oxidation state of +2.
- Halogens (Group 17) almost always possess an oxidation state of -1.
- Hydrogen usually has an oxidation state of +1.
- Oxygen typically bears an oxidation state of -2, except in peroxides like H2O2, where its state is -1.
When assessing molecular compounds, start by assigning oxidation states to hydrogen (+1) or oxygen (-2 or -1) atoms. Then, proceed to assign oxidation states for the remaining atoms. Stoichiometric coefficients must be considered when calculating total ionic charge within compounds. Ensure that the sum of individual atom oxidation states, multiplied by their associated stoichiometric coefficients, equals the net charge of the molecule.
In a neutral compound, the sum of constituent atoms' oxidation states should be zero. For example, consider the oxidation states for potassium permanganate (KMnO4):
K (+1) + Mn (unknown variable "x") + O (-2) x 4 = 0
Solving this equation algebraically yields x = +7. Consequently, in potassium permanganate, manganese (Mn) has an oxidation state of +7, potassium (K) has an oxidation state of +1, and oxygen (O) has an oxidation state of -2.
FIGURE 1: LABELED OXIDATION STATES OF POTASSIUM PERMANGANATE
For an individual ion or a polyatomic ion, the collective oxidation states of its constituent atoms invariably sum up to the overall ionic charge of the compound. Let's illustrate this by assigning oxidation states to the phosphate ion, with the molecular formula PO43− and a net charge of -3.
Oxygen generally maintains an oxidation state of -2, while the oxidation state of phosphorus is undetermined—let's denote it as an unknown variable "x." Employing the molecular formula, we can establish an algebraic equation to find the value of x:
x + (4)(-2) = -3
Solving this equation results in x = +5. Therefore, the oxidation state of phosphorus in the phosphate ion is +5. The oxygen atoms in this compound exhibit an oxidation state of -2.
FIGURE 2: LABELED OXIDATION STATES OF THE PHOSPHATE ION
c) Hydrides
While deviations from the oxidation state rules are infrequent, exceptions may arise due to variations in electronegativity. An example of this is observed with hydrogen, which assumes the role of a hydride when bonded to less electronegative metals (metal hydrides). This behavior results in an oxidation state for hydrogen of -1 rather than its customary +1.
Hydrides, represented by negatively charged hydrogen ions (H-), can be conceptualized as protons with a pair of free electrons. Consequently, gaining a hydride ion invariably constitutes a reduction, whereas losing a hydride ion leads to oxidation. In biochemical contexts, it is often convenient to view reduction as the gain of a bonded hydrogen atom and oxidation as the loss of a bonded hydrogen atom.
Compounds serving as reducing agents, undergoing oxidation while reducing another compound, often feature multiple hydrides. Notable examples include NaBH4 and LiAlH4 in carboxylic acid organic chemistry. The presence of a metal ion and numerous hydrides characterizes these compounds. Hydride ions function as "free electrons," readily departing from the metal complex to effect reduction in another compound, leaving the reducing agent itself in its oxidized state.
Hydrides play a crucial role in cellular processes, especially concerning electron-carrying molecules like NADH and FADH2. In the context of carbohydrate metabolism, NAD+ undergoes reduction to NADH by incorporating a hydride ion. This reduction reaction is consistently linked to the oxidation of another molecule, such as a hydrocarbon substrate.
For a review of this topic, see our comprehensive guide on cell metabolism.
----
Part 3: Applications of redox reactions
a) Balancing redox reactions
Now, let’s look at how redox reactions are applied in different scenarios. Remember that the reduction of one compound is intricately linked to the oxidation of another. How do we quantify the extent of reduction or oxidation for a specific substance?
Balancing a redox reaction involves multiple steps, more than a regular chemical reaction. However, redox reactions adhere to the same fundamental principle of mass conservation.
To balance redox reactions, start by assigning oxidation numbers to each atom participating in the reaction. Next, rewrite the reactants and products as two half-reactions, one being an oxidation reaction and the other a reduction reaction. Within each half-reaction, ensure atoms are balanced in order to comply with the law of conservation of mass. In cases involving oxygen and hydrogen, balance oxygen atoms by adding water on the opposite side and hydrogen atoms by adding H+ on the opposite side. Introduce electrons or multiples of electrons (e-) as needed to maintain charge balance. Multiply each half-reaction by the electrons to facilitate eventual cancellation. Ultimately, combine the two half-reactions to achieve a balanced redox equation.
Listed our, here are the steps:
- Determine oxidation numbers.
- Separate the reaction into half-reactions by distinguishing oxidation and reduction components.
- Balance the mass through coefficients, and if necessary, by introducing water and H+.
- Balance charge by incorporating electrons.
- Balance electrons by multiplying each reaction to yield a net electron count of zero.
- Combine the half-reactions.
Let's illustrate this process with an example:
Compose and balance an equation for the oxidation of copper to Cu2+, combined with the reduction of Ag+ to elemental silver.
Begin by establishing oxidation numbers for each element. The oxidation state for copper shifts from 0 to 2+, and for silver, it changes from 1+ to 0. Copper loses electrons, leading to a more positive oxidation number, while silver gains electrons, yielding a less positive oxidation number. Consequently, copper undergoes an oxidation reaction, and silver undergoes a reduction reaction. The two half-reactions are outlined below:
----
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.