Nomenclature for the DAT
/Learn key DAT concepts related to IUPAC naming rules, plus practice questions and answers
everything you need to know about iupac naming rules and nomenclature for the dat
Table of Contents
Part 1: Introduction to nomenclature
Part 2: IUPAC naming rules
a) Basics
b) Alkanes, alkenes, alkynes
Part 3: Naming functional groups
a) Alkyl halides
b) Oxygen-containing groups
c) Nitrogen-containing groups
d) Aromatic groups
e) Complex compounds
Part 4: High-yield terms
Part 5: Questions and answers
----
Part 1: Introduction to nomenclature
Naming organic compounds is a fundamental topic in organic chemistry. Nomenclature provides a method for chemists to communicate the specific structure of organic compounds. Using proper nomenclature, a compound’s structure can be deduced with no need for a visual reference. The DAT commonly tests your ability to correctly name organic compounds. This guide will teach you everything you need to know about nomenclature for the exam. When you understand the content, test yourself with DAT-style practice questions at the end.
----
Part 2: IUPAC naming rules
a) Basics
On the DAT, you’ll likely be tasked with selecting the correct name for at least one or two organic compounds. Compounds can either be referred to by their common name or their IUPAC name. Common names are an effective way to name simple organic compounds, and organic chemists frequently use these names. Examples of common names include acetic acid, glucose, and toluene. IUPAC names, on the other hand, are an efficient way to name complex organic compounds that can’t be easily communicated with a common name. Some compounds have both common and IUPAC names. IUPAC naming rules were set by an international chemistry union. For the DAT, you need to know how to determine the IUPAC name of a compound, so these names will be the primary focus of this guide.
b) Alkanes, alkenes, alkynes
Recall that alkanes, alkenes, and alkynes are all simple hydrocarbon compounds. Alkanes contain only hydrogen and carbon atoms, all with only single bonds. Alkenes have at least one carbon that is double bonded to another carbon, while alkynes have at least one triple bonded carbon atom. These types of hydrocarbons have a unique suffix that is used in naming. Alkanes are named with the suffix -ane, alkenes are -ene, and alkynes are -yne. Notice that these suffixes are the same suffixes found in the names themselves, so they shouldn’t be too hard to remember.
Organic compounds are named based on the number of carbon atoms they contain. The table below outlines the prefixes used depending on how many carbon atoms are in a compound.
| |
|
Now, let’s practice actually naming a few organic compounds. The organic compound below has 4 carbon atoms and is an alkane. The prefix for four is but- and the suffix for alkane is -ane, so this compound is butane. Butane is both the common and IUPAC name.
FIGURE 1: BUTANE
If a double bond is added to this compound, it is no longer butane. Because it is an alkene, the name changes to butene. This is the common name, but it can be named more specifically using IUPAC rules. For example, both of the compounds below are butene, but notice that the double bond is in a different location.
FIGURE 2: BUTENE WITH NUMBERED CARBONS
To specifically name these butene compounds, we need to use the IUPAC name. Start by numbering the carbons from one end to the other as shown in the image above. You can begin numbering the carbons from either side, but start from the side that will result in the double bond being the lowest number. For the left butene, the double bond spans from carbon 1 to carbon 2. If instead you had numbered the carbons from right to left, the double bond would begin on carbon 3. Numbering the compound this way would be incorrect because it does not follow IUPAC rules. The IUPAC name for the left compound is 1-butene or but-1-ene. The number 1 is given in the name to convey that the double bond begins on the first carbon. The name for the compound on the right is trans-2-butene, or trans-but-2-ene. This is its IUPAC name because it is in the trans conformation, and the double bond begins on the second carbon.
For more complex alkenes, you may need to use the E/Z naming convention. If an alkene has a hydrogen atom attached to a double-bonded carbon atom, use trans- or cis-. However, if there are no hydrogen atoms attached to the double-bonded carbon and all substituents are different, use E- and Z-. Both E/Z and cis/trans are placed at the start of a compound's name, followed by a hyphen. For a review of E and Z configuration, see our guide on stereochemistry.
Try naming the hydrocarbon below:
FIGURE 3: UNNAMED COMPOUND
The name for this compound is 1-butyne because there are four carbon atoms and the triple bond is on the first carbon. This compound is an alkyne, so the suffix -yne is used.
Not all hydrocarbons are as simple as the examples we’ve looked at so far. Compounds can be cyclized or branched. For cyclized compounds like the one pictured below, name them as you would any other hydrocarbon, but add cyclo- at the beginning of the name. The compound below has 6 carbons and only single bonds, so its name would be hexane. However, because it forms a ring, its name is cyclohexane.
FIGURE 4: CYCLOHEXANE
Branched hydrocarbons contain alkyl groups. Alkyl groups are alkanes with one less hydrogen than normal, due to the fact that they are attached to a longer hydrocarbon. Alkyl groups are named using the prefix for number of carbons, followed by the suffix -yl. If a branched hydrocarbon has a singular carbon attached to the main chain, this carbon is referred to as an methyl group. Some of the most common alkyl group names are explained in the figure below.
FIGURE 5: NAME, CONDENSED STRUCTURE FORMULA, LINE STRUCTURE FOR COMMON ALKYL GROUPS. R REPRESENTS THE REST OF THE COMPOUND.
Let’s look at a few examples to better understand how to name compounds with alkyl groups.
FIGURE 6: UNNAMED COMPOUND
To name this compound, you must determine how many carbon atoms are in the longest continuous string of carbons. The longest continuous carbon chain is known as the parent chain, and the number of carbon atoms will determine what the compound is named. For this example, the longest continuous chain of carbons contains eight atoms. There are no double or triple bonds, so this compound—excluding any substituents—is octane. Now, we must account for the alkyl group attached to the third carbon. This alkyl group consists of only one carbon atom, so it is called a methyl group.
To fully name this compound, number the carbon atoms on the parent chain. Begin on the side that will give the methyl group the lowest possible number. For this compound, beginning on the left side will assign the methyl group to the 6th carbon, while starting from the right will assign it to the 3rd carbon. The full name of this compound is 3-methyloctane.
FIGURE 7: 3-METHYLOCTANE. RED NUMBERS ARE THE CORRECT WAY TO NUMBER THIS COMPOUND, BLUE NUMBERS ARE INCORRECT.
What about for compounds that have multiple alkyl groups like the one pictured below?
FIGURE 8: UNNAMED COMPOUND
Again, start by naming the longest continuous string of carbon atoms. For this example, there are five carbon atoms, so this compound is pentane. Next, number the pentane carbons. The two methyl groups are attached to carbons two and four. To account for multiple alkyl groups of the same name, use the following table of prefixes:
Because there are two methyl substituents, we must add the prefix di- before methyl. The full IUPAC name of the compound above is 2,4-dimethylpentane.
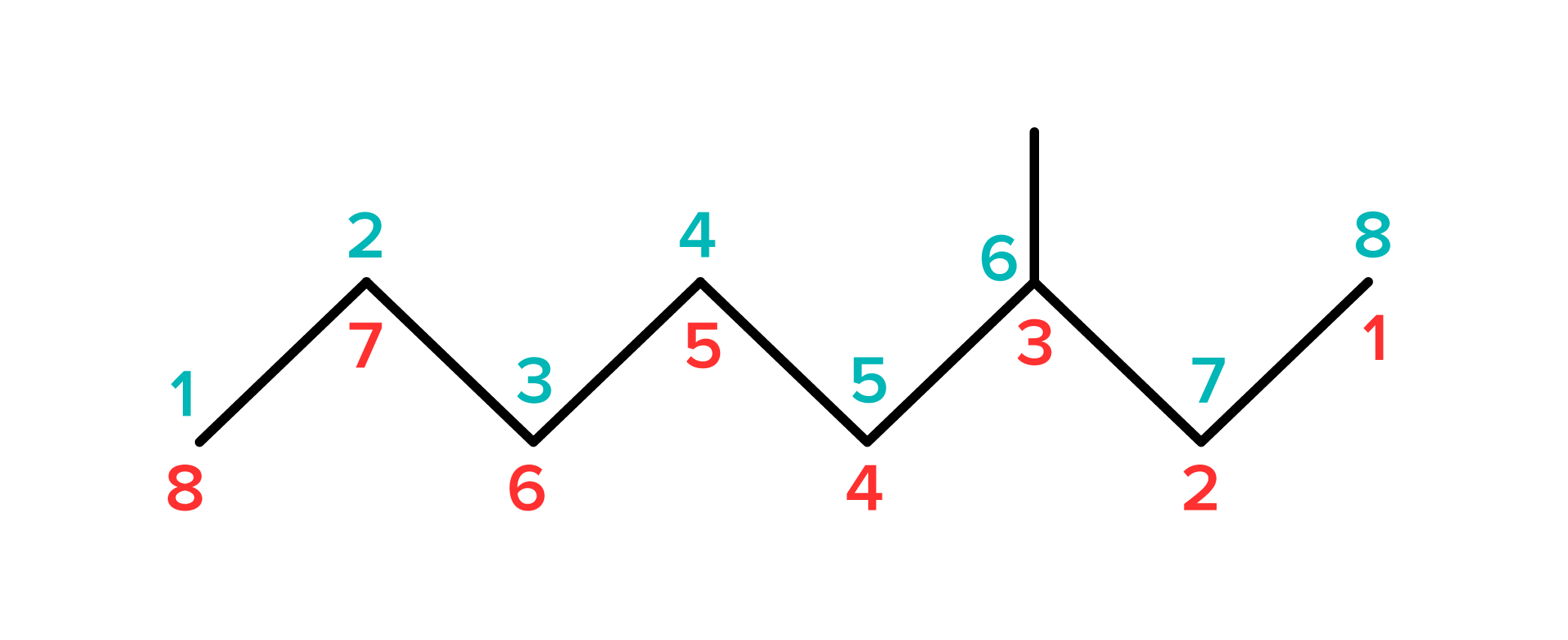
For organic compounds with multiple alkyl groups, the groups are ordered alphabetically. Let’s name the compound below as an example.
FIGURE 9: UNNAMED COMPOUND
Start by determining how many carbons are in the parent chain. This compound’s longest string has 6 carbon atoms, the name of the parent chain is hexane. Next, number the carbon atoms such that all substituents have the lowest possible number. There are two methyl groups on carbons two and four, and an ethyl group (named ethyl because there are two carbons) on the third carbon. This compound's name is 3-ethyl-2,4-dimethylhexane.
----
Part 3: Naming functional groups
a) Alkyl halides
Alkyl halides can be named according to the prefixes listed in the table below. Alkyl halides are the lowest priority substituent group in terms of naming. Don’t worry if you don’t know what the priority is referring to, it will be covered later in section e.
Let's name the alkyl halide below.
FIGURE 10: UNNAMED COMPOUND
Start by naming the chain of carbon atoms. There are seven carbons, so start with heptane. Now, number the carbons from left to right or right to left, such that both substituents have the lowest number. Starting from the left assigns 2 to the bromine group and 5 to the chlorine group. The name of this compound, then, is 2-bromo-5-chloroheptane.
b) Oxygen-containing groups
To name compounds that contain oxygen atoms, use the following table.
These suffixes are placed at the end of the name of the carbon chain. For example, the simple carboxylic acid below is named propanoic acid. Prop- signifies that there are three carbon atoms. -an- is the hydrocarbon suffix with the last letter removed, and is used because all carbon atoms in the parent chain are connected to each other by single bonds. -oic acid is given because the compound is a carboxylic acid.
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.