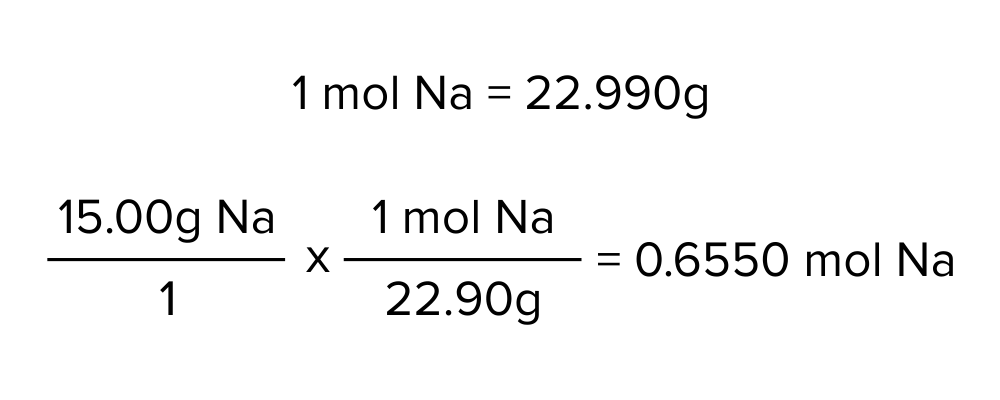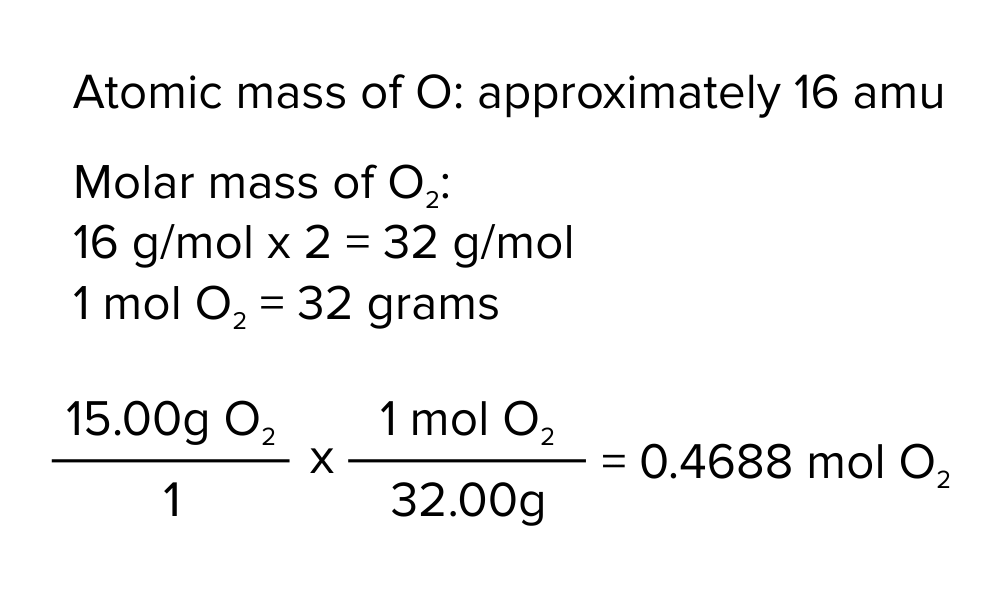Molecules and Stoichiometry for the MCAT: Everything You Need to Know
/Learn key MCAT concepts about molecules and stoichiometry, plus practice questions and answers
(Note: This guide is part of our MCAT General Chemistry series.)
Table of Contents
Part 1: Introduction to molecules and stoichiometry
Part 2: Moles
a) Definition of a mole
b) Molecular mass
Part 3: Formation of molecules
a) Representations of molecular formulas
b) Types of chemical reactions:
Part 4: Stoichiometry
a) Balancing equations
b) Determining theoretical yield
Part 5: High-yield terms
Part 6: Passage-based questions and answers
Part 7: Standalone questions and answers
-----
Part 1: Introduction to molecules and stoichiometry
Molecules may be difficult to conceptualize because they cannot be easily seen. For instance, you may be able to see individual grains of table salt, but you cannot visualize a molecule of salt: the smallest possible unit of salt that can be identified as such. Even then, the salt molecule can be further broken down into atoms such as sodium and chlorine atoms.
Stoichiometry allows us to quantify and understand such quantities by employing conversion factors centralized around the unit of mole. In chemistry, we often rely on stoichiometry to relate quantities of reactants and products. Thus, stoichiometry allows us to balance chemical equations according to laws of conservation.
Understanding chemical equations and stoichiometry is crucial to performing chemistry-related calculations. While these calculations may be tricky, using scratch paper and the Periodic Table during the official MCAT is permitted.
Throughout this guide, several important terms are highlighted in bold. At the end of this guide, there are also several AAMC-style practice questions for you to test your knowledge with.
Let’s begin by understanding moles.
-----
Part 2: Moles
a) Definition of a mole
How do we quantify reactants and products? The amount of a material that is present may be defined in terms of mass, numbers of particles, or some other unit.
It may be difficult to count the numbers of particles within a quantity of reactants or products. This is because the particles are very small and may be atoms or molecules. (For a review on the structure of the atom, be sure to refer to our guide on atomic and nuclear physics.) Instead, a unit called a mole (mol) is used to define these quantities.
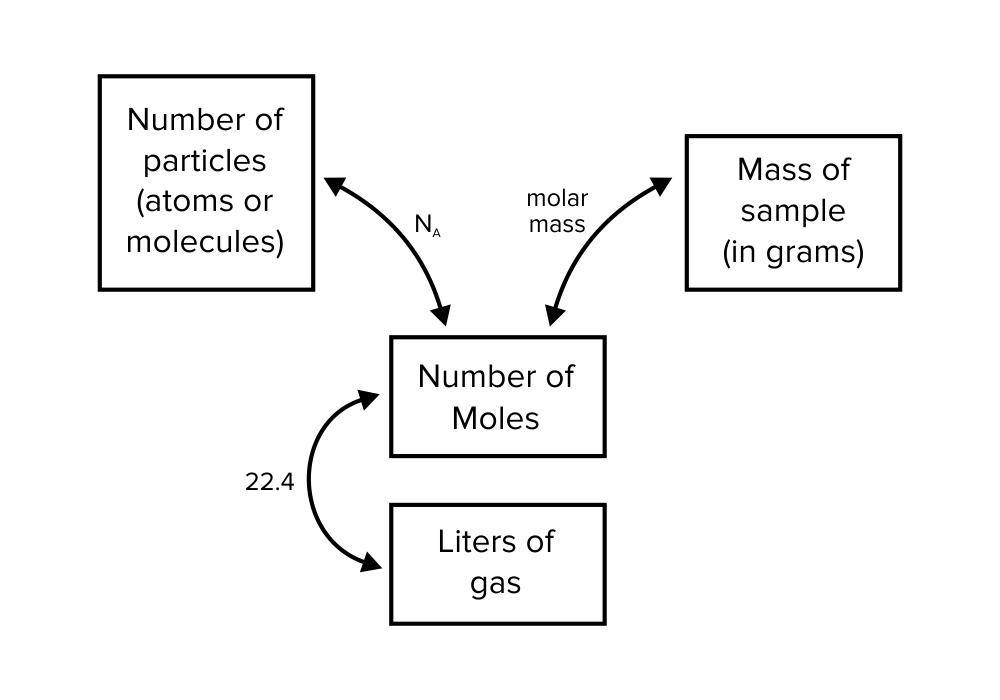
Figure: Different ways of expressing the same quantity of a substance.
A mole can also be defined in the context of gaseous substances. One mole of a gaseous compound occupies 22.4 L under standard temperature and pressure (STP). In chemistry, standard temperature and pressure refer to the following: a temperature of 273.15 K and an absolute pressure of 100 kPa (equal to 1 bar).
b) Molar mass
The atomic or molecular mass of a substance is used to convert the number of moles that are present to the mass in grams. For this reason, it is also referred to as molar mass or molecular weight.
Determining the molar mass of an atomic element is relatively simple. For the case of monoatomic elements, the molar mass of the element is simply the atomic mass. The atomic mass can be found right below the element’s symbol on the periodic table. For instance, Na (sodium) has an atomic mass of 22.99 AMU. Thus, we can use this number to convert the mass of a known quantity of sodium to the number of moles in that sample.
Figure: Converting 15.00 g of sodium (Na) to the corresponding quantity of moles.
Let’s take oxygen as an example. The atomic mass of oxygen is 15.999, approximately 16 AMU. However, oxygen in its natural state is present as a diatomic molecule (O2). Thus, to determine its molar mass, we must multiply this quantity by the number of atoms present in the molecule.
Figure: Converting 15.00 g of diatomic oxygen (O2) to the corresponding quantity of moles.
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.