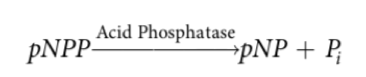MCAT Chemistry Practice Questions
/Prepare for MCAT chemistry questions with practice problems
(Note: This resource also appears in our MCAT Ultimate Guide.)
----
Introduction
MCAT Chemistry Practice Passage #1
MCAT Chemistry Practice Passage #2
MCAT Chemistry Practice Passage #3
MCAT Chemistry Practice Questions (Standalone)
----
Introduction
As a premed, you’ve undoubtedly had to wade your way through general chemistry and organic chemistry. Some of you may have even ventured into the murky waters of physical chemistry, inorganic chemistry, or advanced general chemistry. Your hard work in those classes will pay off as you begin studying for the MCAT.
While your study schedule should be optimized to strike an optimal balance between content and practice, you’ll still need to be aware of the important pieces of general chemistry and organic chemistry content.
The MCAT is a hard exam, so in addition to knowing the content, you’ll need to know how to solve practice problems. In fact, the test writers generally take a scientific article, include a few figures, and ask you questions that require you to draw on information from the passage and outside content knowledge.
So, what’s the best way to improve your MCAT score? Practice! Here, we’ll test your chemistry knowledge using MCAT-style passages. Use the following three chemistry passages and five standalone questions to test your readiness for MCAT-style passages. Each explanation for the passage-based questions will have suggestions for what you should review if you miss a question. Good luck!
----
MCAT Chemistry Practice Passage #1
Most biochemical reactions depend on the pH value of the aqueous environment and many are strongly favored to occur in an acidic environment. A non-invasive control of pH to tightly regulate such reactions with defined start and end points is a highly desirable feature in certain applications.
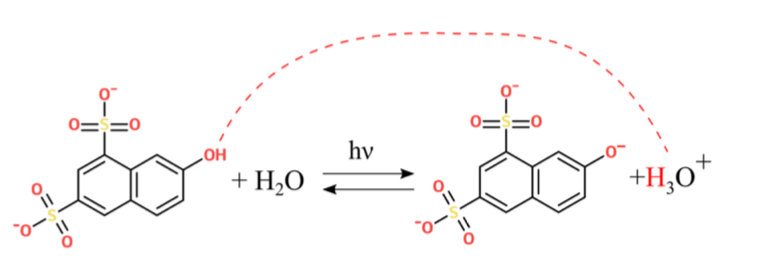
Researchers report a novel optical approach to reversibly control a typical biochemical reaction by changing the pH and using acid phosphatase as a model enzyme. The reversible photoacid G-acid functions as a proton donor, changing the pH rapidly and reversibly by using high power UV LeDs as an illumination source in the experimental setup. The researchers found that the reaction can be tightly controlled by switching a light on and off, making the technology applicable to a wide range of other enzymatic reactions, thus enabling miniaturization and parallelization through non-invasive optical means. Figure 1 displays the structure of the G-acid.
Figure 1. Light activated deprotonation of G-acid.
In order to use the photoacid in a wide range of biochemical applications, the researchers ensured that it met certain requirements, such as solubility in water, low toxicity, and excitability with commonly available, inexpensive illumination sources, such as LEDs. LEDs have several advantages compared to more conventional illumination sources: They are more efficient, have a long life-time, a compact size, and high reliability.
The researchers chose an acid phosphatase as a model enzyme to further study the system. The activity optimum of APs typically lies at an acidic pH of 4.5–5.5. APs non-specifically catalyze the hydrolysis of monoesters to produce inorganic phosphate under acidic conditions. In experiment A, researchers used the acid phosphatase from potato (EC 3.1.3.2), which is active between pH 4–7, with an activity optimum at a pH of 5–5.3. At an alkaline pH of 8, the activity of EC 3.1.3.2. is several orders of magnitude lower.
To assay EC 3.1.3.2. activity, researchers used p-nitrophenyl phosphate (pNPP) assay. The pNPP assay is a colorimetric assay in which the substrate pNPP is hydrolyzed by acid phosphatase into the product p-nitrophenol (pNP) + Pi in purified HPLC grade water as shown by Figure 2.
Figure 2. Reaction scheme for pNPP assay.
1. Which of the following best describes the role of water in the deprotonation of the G-acid?
A) Acid
B) Catalyst
C) Base
D) Aprotic solvent
A) 2.0 x 1022
B) 1.5 x 1022
C) 1.0 x 1022
D) 0.5 x 1022
3. Which of the following functional groups is NOT found on the protonated G-acid?
A) Alcohol
B) Benzene
C) Sulfonate
D) Sulfite
4. Researchers are likely to observe which of the following during a pNPP assay for EC 3.1.3.2. when pH is lowered to 5?
A) Cleavage of EC 3.1.3.2.
B) An increase in inorganic phosphate levels
C) A decrease in sulfonate levels
D) Decrease in EC 3.1.3.2. activity
Answers and explanations for MCAT Chemistry Practice Passage #1
1. The correct answer is C. Water is a base as it accepts a proton from the G-acid (choice C is correct; choice A is incorrect). Water is not a catalyst in this reaction as it is not regenerated or used to lower the activation energy of the reaction. Water is a reactant (choice B is incorrect). Water is a protic solvent (choice D is incorrect).
Review acids and bases, catalysts, and protic versus aprotic solvents.
Review E = hv equation and MCAT math.
3. The correct answer is D. An alcohol (-OH) group is present on the protonated G-acid (choice A is incorrect). A benzene ring (aromatic 6-carbon ring) is present within the protonated G-acid (choice B is incorrect). A sulfonate group contains a sulfur atom bound to an R-group (carbon in this case), two double bonds to oxygen, and a single bond to a negatively charged oxygen. There are two sulfonate groups present on the protonated G-acid (choice C is incorrect). A sulfite group contains three oxygens and two negative charges, which is not found on the protonated G-acid (choice D is correct).
Review functional groups.
4. The correct answer is B. When pH is lowered to 5, the EC 3.1.3.2. enzyme is in its optimal pH range according to the passage and activity would thereby increase (choice D is incorrect). Since EC 3.1.3.2. is an acid phosphatase, an increase in activity would lead to increased conversion of pNPP to pNP and inorganic phosphate, or Pi (choice B is correct). The passage does not indicate that the enzyme itself would be cleaved (choice A is incorrect). Sulfonate is attached to the G-photoacid and not directly involved in EC 3.1.3.2. activity (choice C is incorrect).
Review figures from the passage and draw out the trail of logic to arrive at the correct answer. An example trail of logic is: pH -> 5 -> EC 3.1.3.2. -> increased enzyme activity -> increased conversion of pNPP to pNP and Pi -> increased Pi
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.