Important Functional Groups for the MCAT: Everything You Need to Know
/Learn key MCAT concepts about important functional groups, plus practice questions and answers
(Note: This guide is part of our MCAT Organic Chemistry series.)
Table of Contents
Part 1: Introduction to functional groups
Part 2: Oxygen-containing groups
a) Alcohols
b) Aldehydes and ketones
c) Carboxylic acids
d) Carboxylic acid derivatives
Part 3: Nitrogen-containing groups
a) Amides and amines
b) Imines, and enamines
c) Cyanohydrins
Part 4: Hydrocarbon functional groups
a) Hydrocarbons
b) Amino acid side chains
c) Aromatic compounds
Part 5: Passage-based questions and answers
Part 6: Standalone questions and answers
-----
Part 1: Introduction to functional groups
To gain an understanding of organic chemistry, one must first gain an understanding of functional groups. After all, organic chemistry is all about the study of how molecules interact, and functional groups are collections of atoms that confer certain chemical properties to the molecule.
The sheer variety of functional groups can be overwhelming, but don’t worry if you aren’t familiar with them yet! This guide will review the major functional groups covered on the MCAT. As you work through the guide, it will be most helpful to determine if these functional groups are electrophilic or nucleophilic. (For a review of the function of electrophiles and nucleophiles, be sure to refer to our guide on the fundamentals of organic chemistry.) While the MCAT will rarely test you on nomenclature and molecular structure, general trends in electrophilicity, nucleophilicity, and electronegativity will be the most important information to retain for test day.
At the end of this guide, there are also several AAMC-style practice questions for you to test your knowledge against.
Let’s get started!
-----
Part 2: Oxygen-containing groups
a) Alcohols
Alcohols are molecules that contain a hydroxyl group, or -OH.
There are quite a few important properties that alcoholic functional groups have. One of the most interesting properties is their acidity. Alcohols can serve as weak Bronsted acids, with a pKa between 15 and 20. Though they have a high pKa, alcohols are able to donate their protons in basic solutions. When this occurs, the hydroxyl group can now act as a nucleophile.
Alcohols can also participate in hydrogen bonding. Recall that hydrogen bonding relies on the interactions donated by hydrogens bound to other elements of high electronegativity (including oxygen, nitrogen, and fluorine). Because alcohols possess a hydroxyl group with a hydrogen atom bound to an oxygen atom, they can participate in hydrogen bonding. This is an important property to consider because hydrogen bonding can raise the boiling points of many alcohols.
Alcohols also participate in many important synthesis reactions. Alcohols can be converted into higher-level functional groups through oxidation reactions. There are specific reagents that can create aldehydes, ketones, and even carboxylic acids from an alcohol.
Figure: Examples of reactions involving alcohols.
Along with oxidation reactions, alcohols can participate in substitution reactions (Sn1 and Sn2 reactions). In these reactions, the hydroxyl group can serve as a nucleophile, attacking a carbon center and creating a new molecule. Since hydroxyl groups are able to lose a hydrogen ion in basic conditions, these substitution reactions proceed well when occurring in a basic solution.
Alcohols can also partake in protection reactions and mesylate/tosylate reactions. Chemists perform these reactions to ensure the hydroxyl group does not react or interfere during the desired synthesis reaction.
This can be accomplished in several ways. Generally, chemists turn hydroxyl groups into ketones or aldehydes that become ketals/hemiketals or acetals/hemiacetals. Additionally, hydroxyl groups can be converted into functional groups that are better leaving groups. Mesylate and tosylate reactions convert hydroxyl groups into mesylates or tosylates, which are good leaving groups. These reactions help facilitate substitution or elimination reactions in which an alcohol must be eliminated.
There is a specific nomenclature used to describe alcohols. To do so, determine the name of the alkane body (more on this later). Then, remove the -e ending. A suffix of -ol is added at the end of the name. A number describing which carbon the hydroxyl group is bound to in the longest chain is added as a prefix. Naming alcohols is as simple as that!
Figure: This alcohol is named 2-pentanol.
b) Aldehydes and ketones
Both aldehydes and ketones are important functional groups due to their electrophilic nature. The oxygen atom pulls electron density away from the carbon atom, creating a partial positive charge on the carbon atom. This creates a vulnerable site that can be attacked by a nucleophile.
Thus, aldehydes and ketones are commonly involved in nucleophilic addition reactions. These reactions yield many additional functional groups that behave similarly.
Ketal/hemiketal and acetal/hemiacetal reactions are used to protect ketones and aldehydes from participating in undesired reactions. These reactions change the structure of the ketone and aldehyde such that they no longer possess a vulnerable, electrophilic carbon atom.
Ketals are the product of ketones going through two moles of alcohol attack, while a hemiketal has only undergone one mole of alcohol attack. There’s a similar relationship between acetals and hemiacetals, the key distinction being that they are formed from aldehydes. These reactions are also reversible, making this method a great way of forming protecting groups.
Figure: Reactions that create ketals/hemiketals and acetals/hemiacetals.
Aldehydes are susceptible to oxidation reactions. Recall that alcohols can be transformed into aldehydes or ketones. Similarly, aldehydes can be converted into carboxylic acids using the same reactions. The oxidation of aldehydes results in functional groups of a higher oxidation state, such as carboxylic acids. (For more information on this, be sure to refer to our guide on oxidation and reduction reactions.)
There are times where adding a base or acid to a ketone can change the structure of the ketone. The reason is that alpha hydrogens are unusually acidic. Alpha hydrogens are hydrogens attached to the carbons adjacent to the electrophilic carbons in a carbonyl. The addition of a base creates a species known as an enolate whereas the addition of small quantities of acid creates a species known as an enol.
When an enol forms, it may spontaneously become a ketone again, which is known as keto-enol tautomerism. The difference between these two species is that a ketone is electrophilic, while an enol is nucleophilic (due to the location of the double bond). Thus, enols tend to attack electrophilic sites.
Figure: An example of keto-enol tautomerization
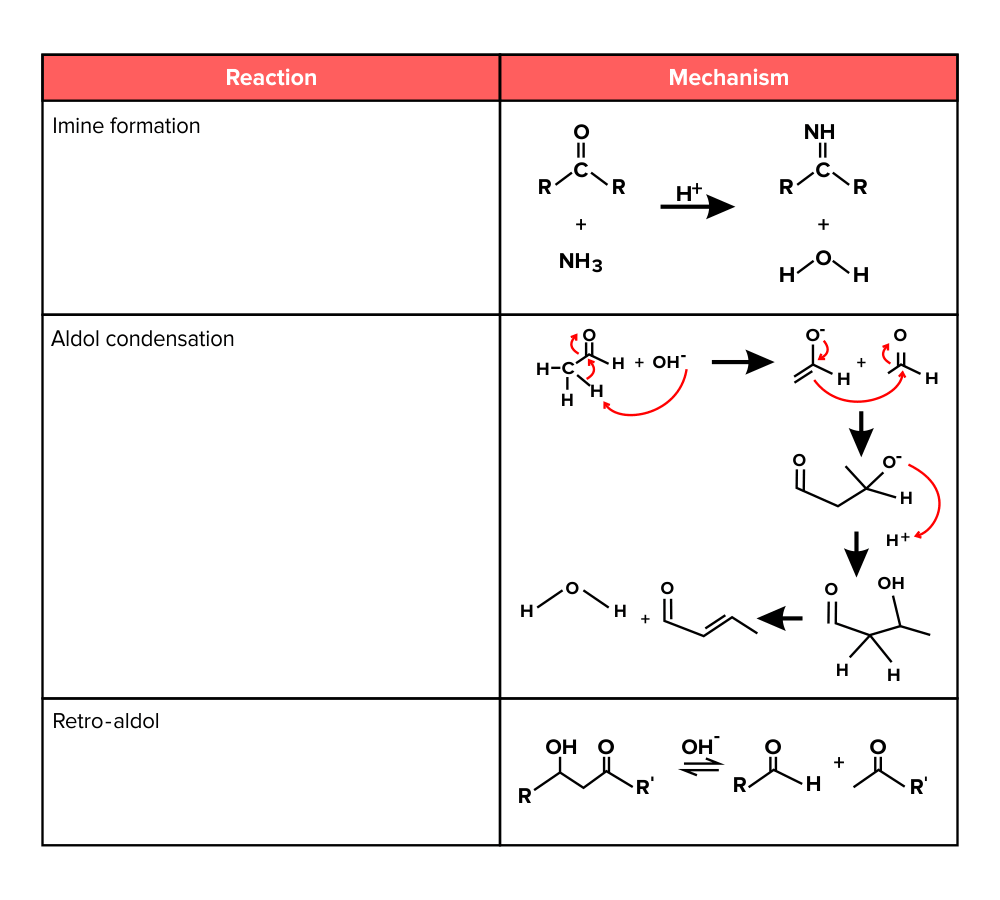
Lastly, an aldol reaction is a way of creating a carbon-carbon bond between two different molecules. A retro-aldol reaction is a method of breaking a carbon-carbon bond in a manner opposite of an aldol reaction. We’ve included the mechanism for the reactions below. Study them carefully to understand how enols and enolates are involved.
Figure: Additional reactions involving aldehydes and ketones.
Gain instant access to the most digestible and comprehensive MCAT content resources available. 60+ guides covering every content area. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.