Chemistry Lab Techniques for the DAT
/Learn key DAT concepts related to chemistry lab techniques and equipment for general chemistry, plus practice questions and answers
everything you need to know about chemistry lab techniques for the dat
Table of Contents
Part 1: Introduction to chemistry lab techniques
Part 2: Equipment and basic techniques
a) Equipment
b) Basic techniques
Part 3: Error analysis
Part 4: Safety
Part 5: Data analysis
Part 6: High-yield terms
Part 7: Questions and answers
----
Part 1: Introduction to chemistry lab techniques
Chemistry laboratory classes are an essential part of the undergraduate curriculum, and while they may seem like there’s no relation to dentistry, there is some similarity. In the lab, there are certain techniques involved to provide high-quality results; the same can be said for dentistry. Additionally, in dentistry, it’s important to understand the names of equipment. Finally, in the lab there are certain dangers and safety is of the utmost importance; in dentistry, we have to be aware of sharps like needles or scalpel blades. Therefore, laboratory techniques provide the foundation for a strong dental understanding. And of course, you need to know chemistry lab techniques for the DAT.
----
Part 2: Equipment and basic techniques
a) Equipment
It is important to know basic laboratory equipment to successfully run an experiment, just as you would want to know basic dental tools for an appointment. Consider the following:
1. Glassware:
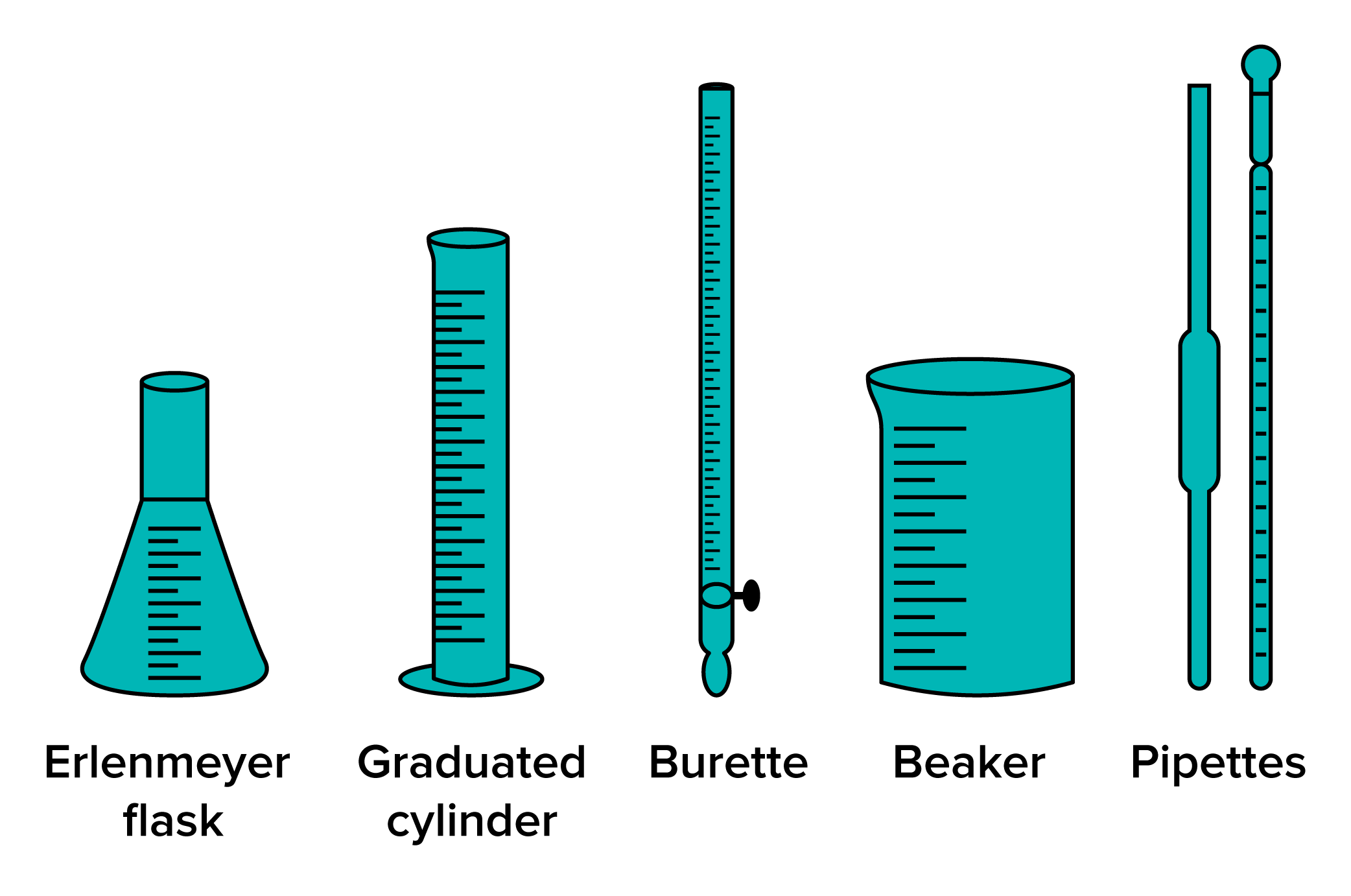
One of the most common types of equipment in general chemistry labs is glassware. This includes beakers, flasks (such as Erlenmeyer flasks and volumetric flasks), test tubes, graduated cylinders, and pipettes. Beakers are used for mixing and heating substances, while flasks are often used for holding and measuring liquids. Graduated cylinders and pipettes are essential for accurate volume measurements during solution preparation and titrations.
FIGURE 1: GLASSWARE USED IN CHEMISTRY LABS
2. Heating devices:
Heating devices like Bunsen burners and hot plates are indispensable in chemistry labs. Bunsen burners provide a direct, adjustable flame used for heating and sterilizing equipment. Hot plates are electrically powered and offer controlled heating for reactions that require more precise temperature regulation.
3. Balances:
Balances are used to measure the mass of solids and liquids with high precision. Analytical balances are sensitive instruments capable of measuring mass to four decimal places, making them ideal for quantitative analysis and preparing precise chemical solutions.
4. pH Meters and electrochemical instruments:
pH meters are used to measure the acidity or alkalinity of solutions accurately. Electrochemical instruments, including voltmeters and conductivity meters, are utilized for analyzing electrochemical reactions and studying solution conductivity.
5. Filtration apparatus:
Filtration equipment such as filter paper, funnels, and vacuum filtration setups are employed for separating solids from liquids. This is crucial for purifying substances or isolating precipitates from solution.
6. Spectrophotometers:
In more advanced labs, spectrophotometers are used to measure the absorbance or transmittance of light by a sample. This technique is valuable for quantitative analysis and studying the properties of substances in solution.
7. Stirring and mixing devices:
Stirring rods, magnetic stirrers, and vortex mixers are essential for mixing solutions and facilitating reactions. Stirring rods are manually operated to mix solutions in beakers, while magnetic stirrers use rotating magnetic fields to stir solutions automatically.
b) Basic techniques
There are certain basic techniques critical for general chemistry. Several are listed below.
Measuring large volumes of liquids requires a graduated cylinder. The graduated cylinder should be rinsed with the solvent you want to measure. To measure the amount of liquid in a graduated cylinder, hold it at eye level and read the meniscus (bottom of the curved liquid surface). Pour liquids slowly and carefully to avoid spills.
FIGURE 2: MEASURING LIQUID LEVEL IN A GRADUATED CYLINDER
You can also use a pipette to draw out smaller amounts of liquid. To use, press the pipette bulb or pipette filler to draw liquids into the pipette. Dispense liquids slowly and steadily, allowing the liquid to drain completely. You can also use micropipettes for precise volume measurements, particularly in biochemical or analytical chemistry experiments.
Next, you may want to pour liquids into a larger container to mix. Hold the container with a steady hand, ensuring a smooth and controlled pour. Use a funnel when transferring liquids into narrow-necked containers to prevent spills. For mixing, use gentle swirling motions to mix solutions without causing splashing or spilling. Avoid excessive agitation, especially with volatile or reactive substances. You can also use a glass stirring rod to mix solutions that require more thorough blending. Rinse the glass rod with distilled water between uses to prevent contamination.
Another critical laboratory technique is filtration. To perform a filtration, fold filter paper into the appropriate shape and place it in a filtration funnel. Ensure the funnel is securely placed in a ring stand or holder. Carefully pour the mixture to be filtered into the funnel. Allow the liquid to pass through the filter paper while retaining the solid.
Next, let’s discuss solids and proper techniques for dealing with them. To measure, use a weighing boat or paper to weigh solids on a balance. Tare the balance to account for the weight of the container, effectively “zeroing out” the balance. To transfer, use a spatula or scoopula to transfer solids between containers or to weigh boats.
Some miscellaneous techniques are listed below. For example use a watch glass to cover beakers during evaporation to prevent splattering and contamination. Additionally, you can use a volumetric flask to accurately prepare a specific volume of a solution.
One of the main general chemistry techniques used is titration. Titrations are covered in depth in our guide on general chemistry acids and bases, but here is the basic procedure. First, prepare standard solutions by accurately measuring a known quantity of solute and adding solvent to a volumetric flask to the calibration mark. Use a wash bottle to rinse any remaining solute from the sides of the flask into the solution. Next, set up the burette, which is a long graduated glass tube used to dispense small amounts of liquids via a stopcock. Rinse the burette with the titrant solution to remove any impurities. Fill the burette with the titrant, ensuring no air bubbles are trapped.
Next, set up the volumetric flask. Place the analyte solution (the solution to be titrated) in a clean Erlenmeyer flask. Add a few drops of an appropriate indicator (e.g., phenolphthalein or methyl orange) to the analyte solution.
Now, you can begin titrating. Slowly add the titrant from the burette into the analyte solution while swirling the flask gently. As the endpoint approaches, add the titrant drop by drop to avoid overshooting the endpoint. The main objective is to observe the color change of the indicator to determine the endpoint of the titration. The endpoint is reached when the color change is permanent (e.g., from pink to colorless with phenolphthalein for acid-base titrations).
----
Part 3: Error analysis
Error analysis is a fundamental process in general chemistry labs, and a high-yield DAT topic. Error analysis involves identifying and quantifying sources of uncertainty to ensure the accuracy and reliability of experimental results. While conducting an experiment, you may introduce different types of errors. These types are random and systematic errors. Random errors are caused by unpredictable fluctuations during measurements. They can be mitigated by taking multiple readings and calculating averages to minimize the impact of outliers. Conversely, systematic errors stem from consistent biases in experimental setup or measurement techniques, often requiring adjustments to equipment calibration or procedures for correction.
Percent error is a measure of the accuracy of an experimental result compared to a known or accepted value. It quantifies the discrepancy between the observed value and the true value, expressed as a percentage of the true value. The formula for percent error is given by:
For example, suppose a student measures the density of a substance in a laboratory experiment and obtains a value of 8.5 g/mL. The accepted value for the density of the substance is 8.9 g/mL. Using the formula for percent error, the percent error in the student's measurement would be calculated as follows:
This indicates that the student's measurement deviates from the accepted value by approximately 4.49%, suggesting a moderate level of error in the experimental result. Percent error is a valuable tool for assessing the reliability of experimental data and identifying areas for improvement in experimental techniques.
Understanding error propagation is essential to assess how errors influence subsequent measurements and overall experimental outcomes. These effects can build up over time, leading to larger and larger margins.
Students use various error analysis techniques, including statistical uncertainty calculations like standard deviation, and graphical representations such as error bars on graphs, to visualize and quantify uncertainties associated with experimental data. By implementing careful experimental techniques, adhering to standard protocols, and conducting repeated trials, students can minimize errors and enhance the reliability of their laboratory findings. Error analysis cultivates critical thinking skills and contributes to the advancement of scientific knowledge by improving the quality and integrity of experimental research in general chemistry.
Precision and accuracy are two essential concepts that define the reliability of experimental measurements. Precision refers to the consistency or repeatability of results, indicating how close multiple measurements of the same quantity are to each other. A highly precise measurement produces values that cluster tightly around a central value. On the other hand, accuracy refers to how close a measured value is to the true or accepted value of a quantity. An accurate measurement reflects minimal systematic error and is in close agreement with the true value. While precision highlights the reproducibility of results, accuracy underscores their correctness. Results can be precise, accurate, or both.
----
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.