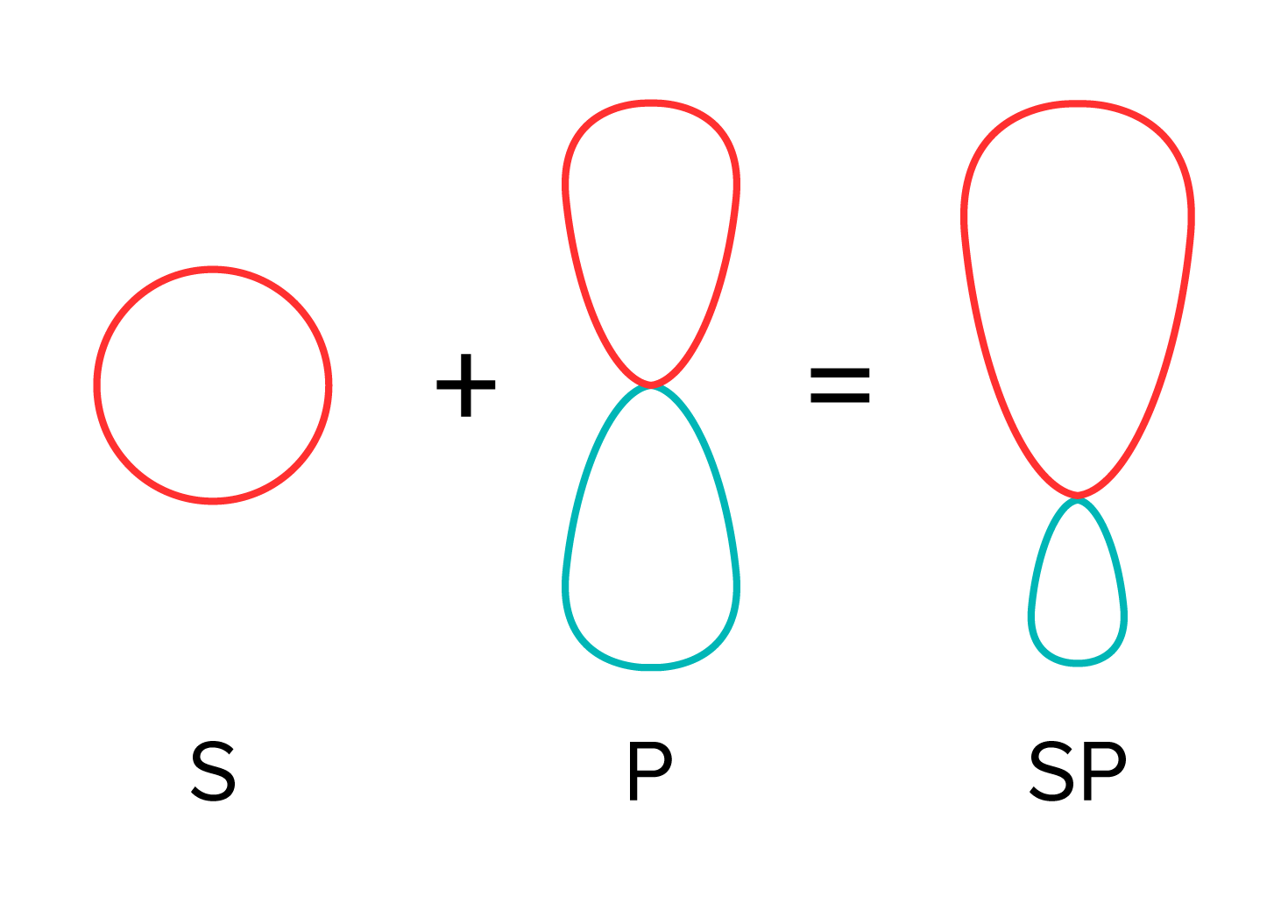Bonding and Aromatics for the DAT
/Learn key DAT concepts related to bonding and aromatics, plus practice questions and answers
Learn key DAT concepts related to bonding and aromatics
Table of Contents
Part 1: Introduction to bonding and aromatics
Part 2: Bonding
a) Bond lengths and angles
b) Atomic and molecular orbitals
c) Resonance
Part 3: Aromatics
a) Aromatic rules
b) Heterocyclic compounds and charges
Part 4: High-yield terms
Part 5: Questions and answers
----
Part 1: Introduction to bonding and aromatics
Bonding is a topic that is covered in both general and organic chemistry. Most of the same principles apply to both topics, and bonding questions can be tested in either section of the DAT. Unique aspects of bonding, like resonance and conjugation, play a role in the formation and stability of aromatic compounds. This guide will teach you everything you need to know about bonding and aromatics for the organic chemistry section of the DAT, with exam-style practice questions and answers at the end.
----
Part 2: Bonding
a) Bond lengths and angles
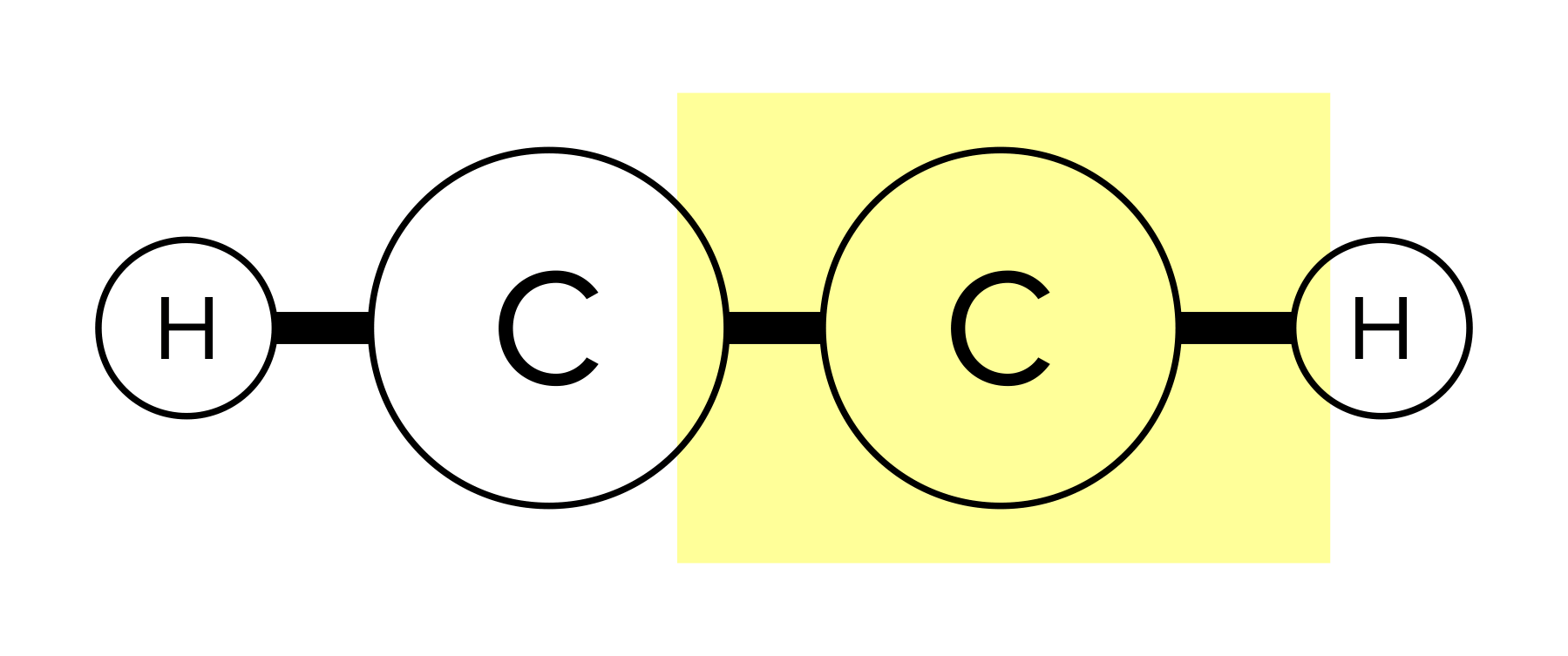
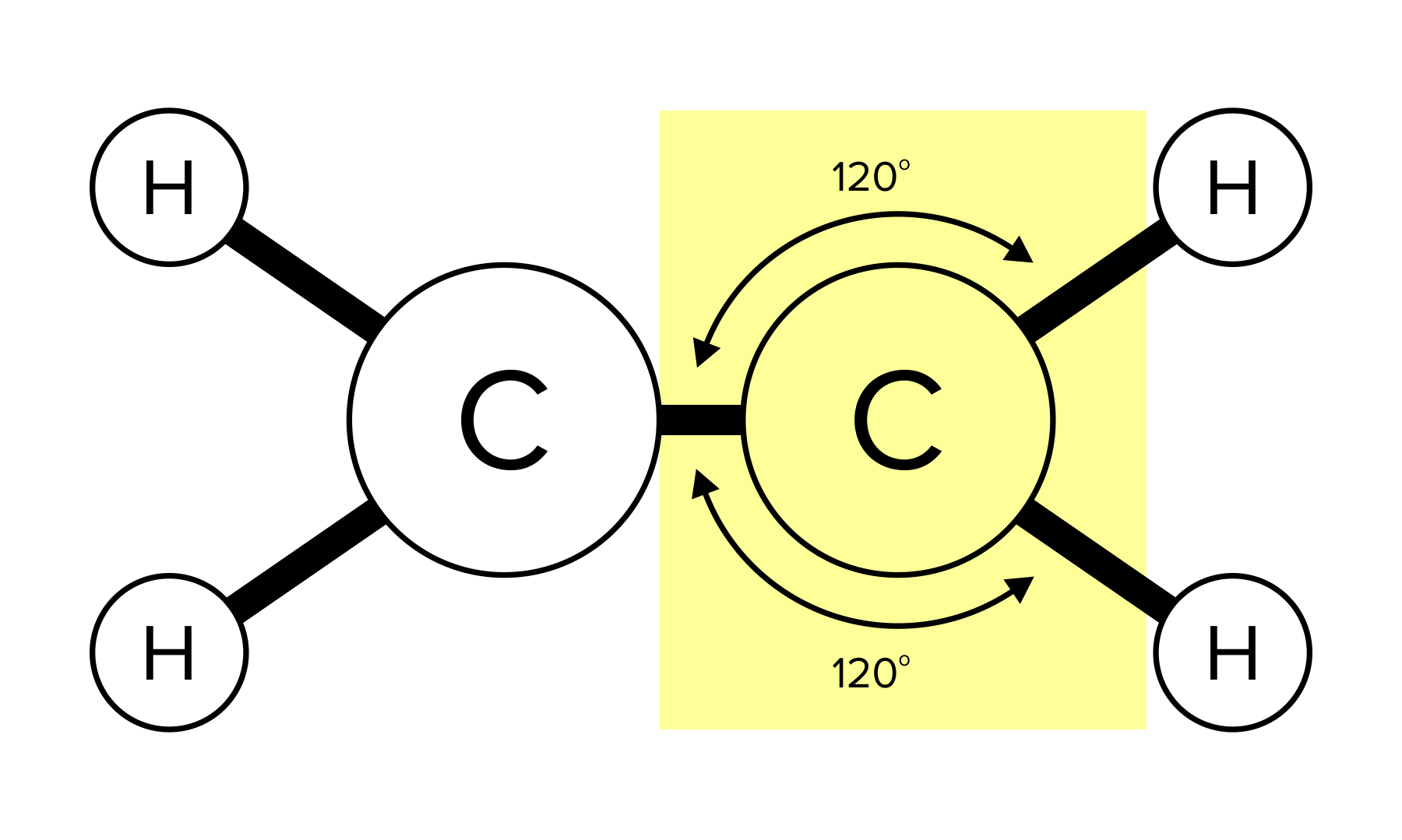
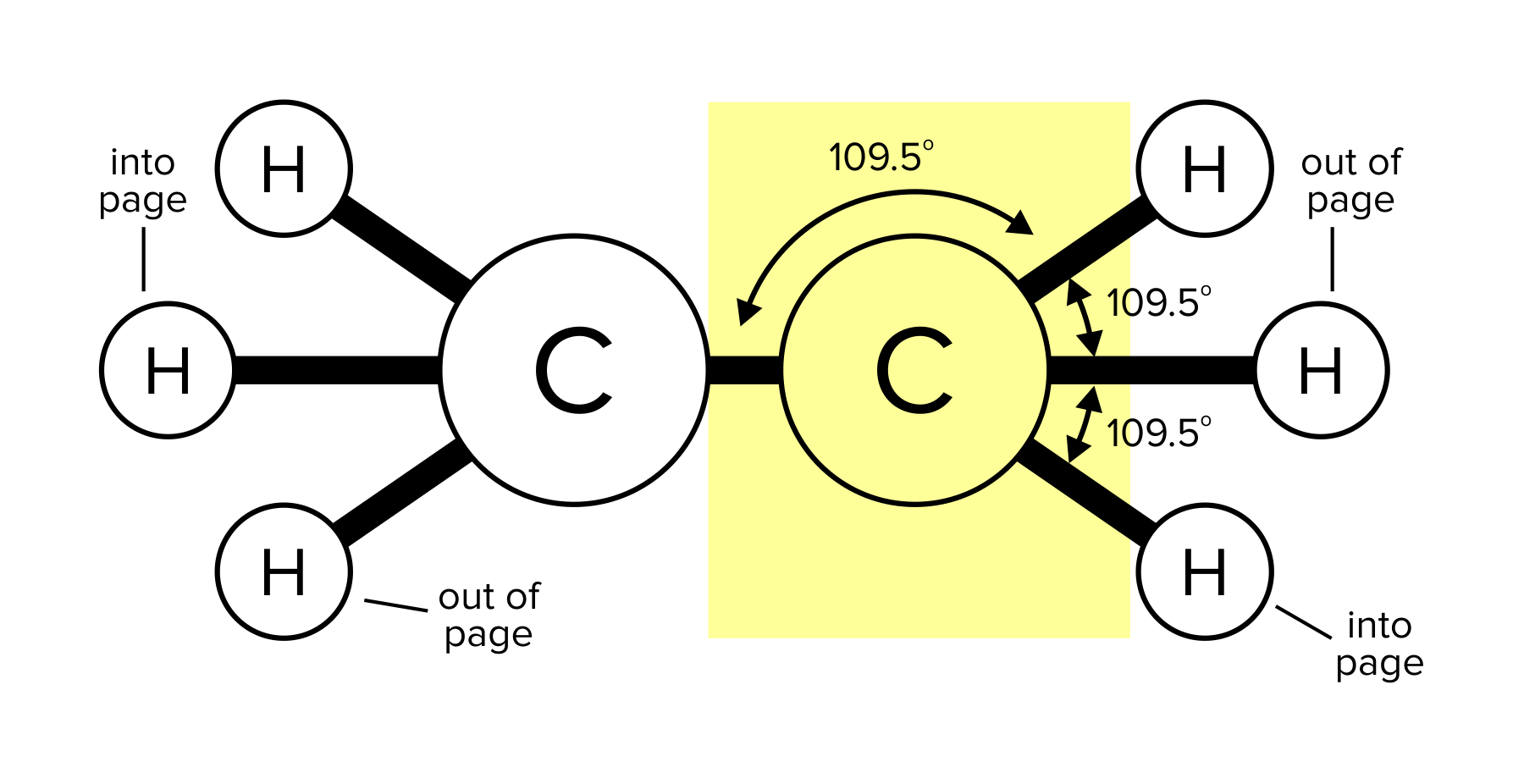
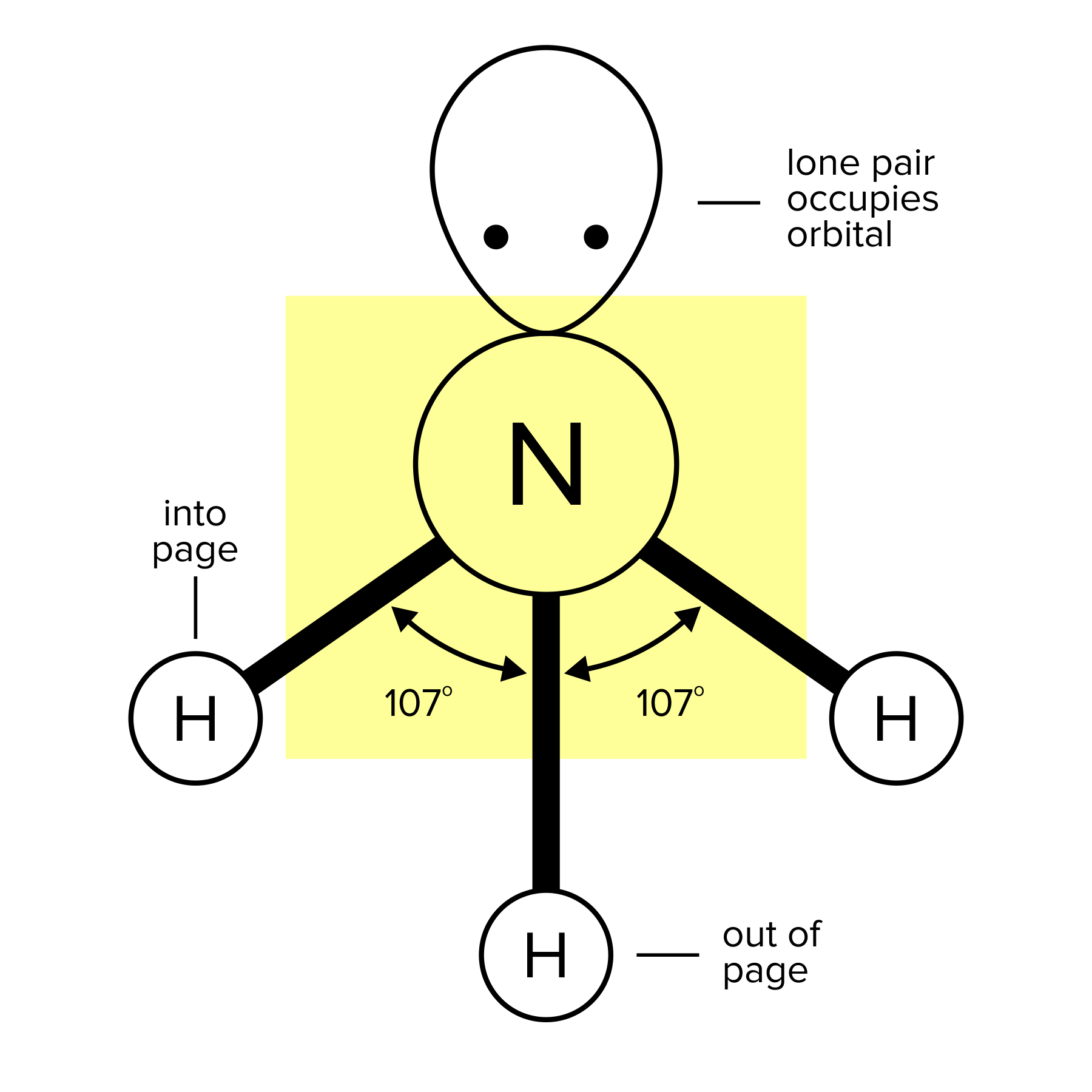
Recall that a bond is the force that holds molecules together. A molecule can have single, double, or triple bonds. Triple bonds are shorter than double and single bonds, while single bonds are the longest of the three. A compound can take different geometrical shapes based on its number of bonds and lone pairs of electrons. These concepts are covered more in-depth in our general chemistry guide on atomic and molecular structure.
| Hybridization | Bond angle | Predicted geometry | Example |
|---|---|---|---|
One atom bonded to two others |
|||
One atom bonded to three others |
|||
One atom bonded to three other atoms and one lone pair |
b) Atomic and molecular orbitals
Atomic orbitals are 3D structures that show the probable location of electrons. Recall that electrons can exhibit wavelike behavior. Atomic orbitals also are a way to describe this behavior. S and p orbitals are the most common type, but an atom can also have d and f orbitals. S and p orbitals on atoms overlap and combine to create hybrid orbitals in a process known as hybridization. Sp orbitals are a combination of one s and one p orbital, while sp2 orbitals are a hybrid of one s and two p orbitals. Sp3 orbitals follow suit, consisting of a combination of one s and three p orbitals.
FIGURE 1: HYBRIDIZATION OF ONE S AND ONE P ORBITAL
Atoms with four single bonds are sp3 hybridized. Atoms with three single and double bonds are sp2 hybridized. This is because one of their p orbitals is used to form the pi bond of the double bond, while the other two p orbitals are combined with the remaining s orbital to create the sp2 hybrid. Atoms with a triple bond and a single bond are sp hybridized, and the hybrid orbitals consist of one s and one p orbital.
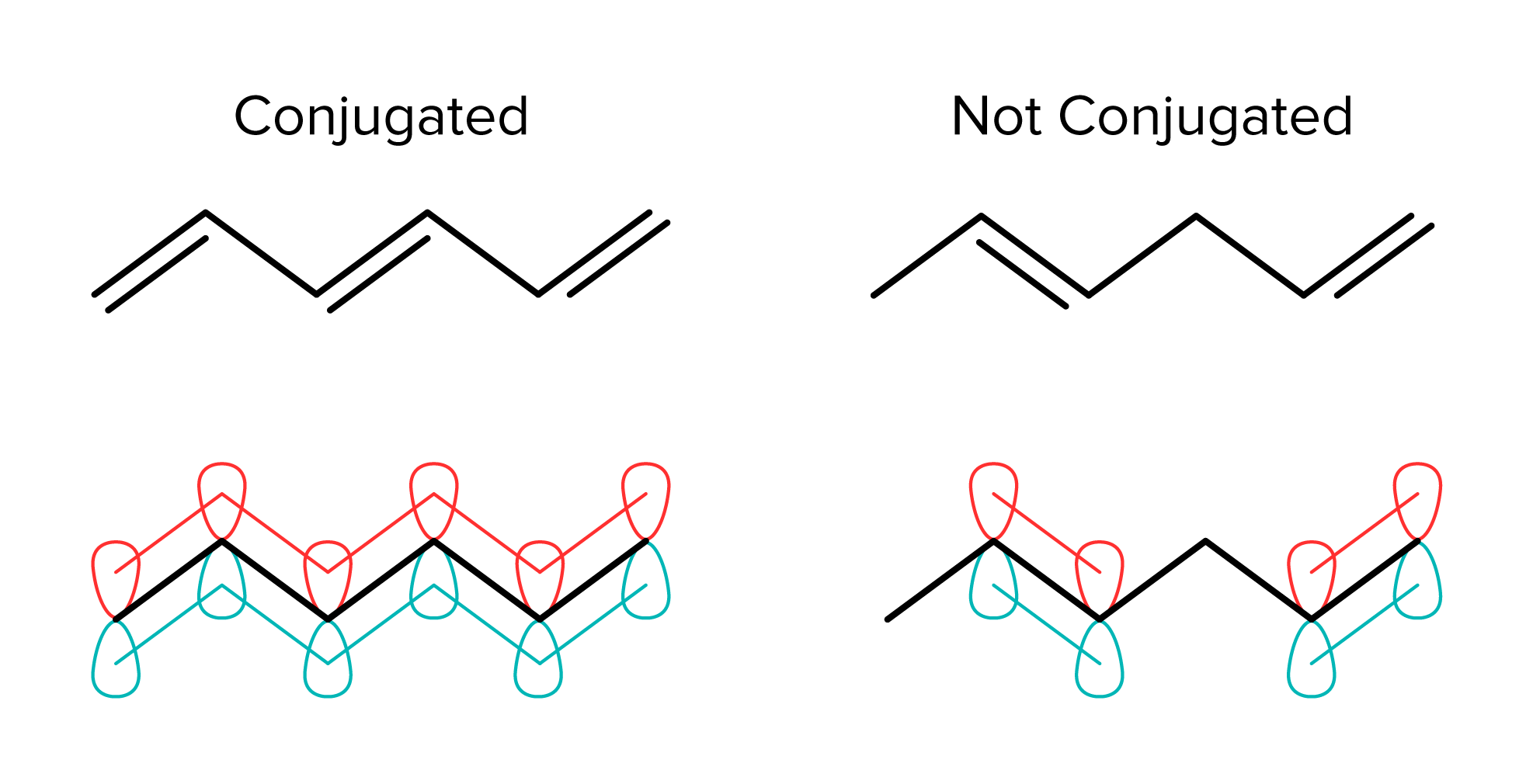
Conjugation occurs in organic compounds that have alternating double and single bonds. Compounds with conjugated bonds are more stable than they would be without conjugation. This stability is due to the p orbitals of the double bonds. When p orbitals of different double bonds are next to each other, they allow pi electrons to delocalize. This means that the electrons are associated with multiple pi bonds, not just one. Delocalization increases the stability of a compound. Conjugation can be interrupted by other atoms, such as in the example on the right. The bonds are not conjugated because an sp3 carbon is located in between the double bonds.
FIGURE 2: ORBITAL CONJUGATION
c) Resonance
Resonance is a way to describe the delocalization of electrons in a compound. Just like with conjugation, resonance involves electrons that are not associated with only one single bond. Resonance structures are a way to represent the delocalization of electrons. Let’s look at ozone as an example of resonance.
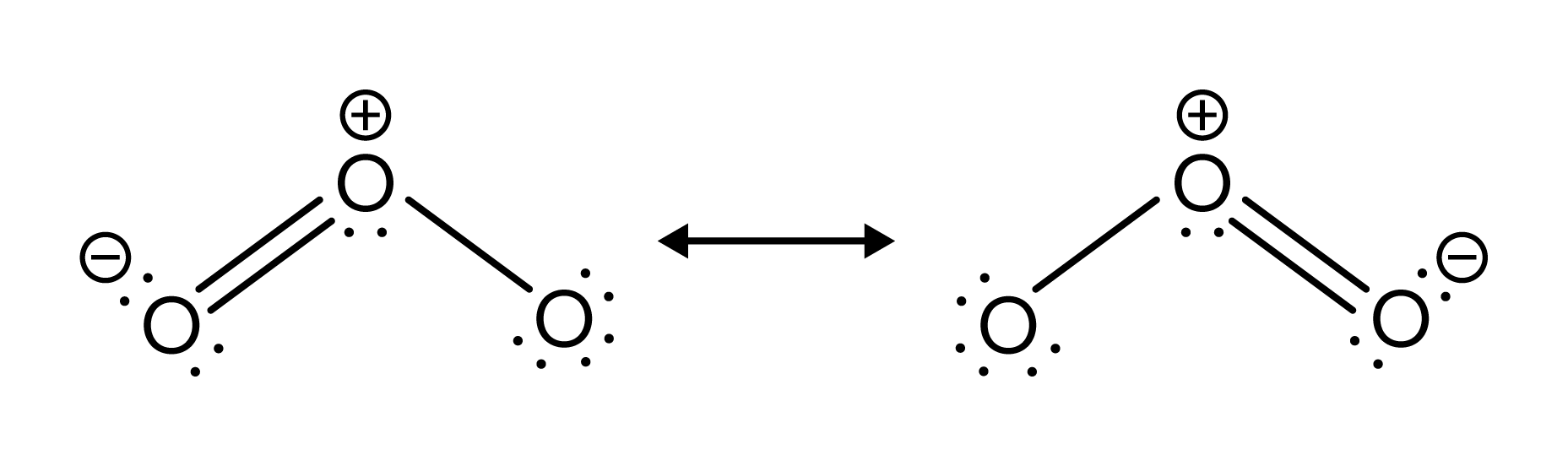
Ozone consists of three oxygen atoms bonded together. In order to follow the octet rule (see our general chemistry guide on atomic and molecular structure for review), six electrons will be involved in bonding. Does this mean that one oxygen atom will always be double bonded while the other is single bonded? Not exactly. One pair of electrons will resonate between both bonds. This resonance is depicted in the visual below.
FIGURE 3: OZONE RESONANCE STRUCTURES
The left structure shows the double bond on the left, while the right structure shows the double bond on the right. In reality, the length of each bond on ozone is the same, so the actual structure is an equal mix of the left and right structures. The double-headed arrow signifies that the real structure of the molecule is in between the two structures shown.
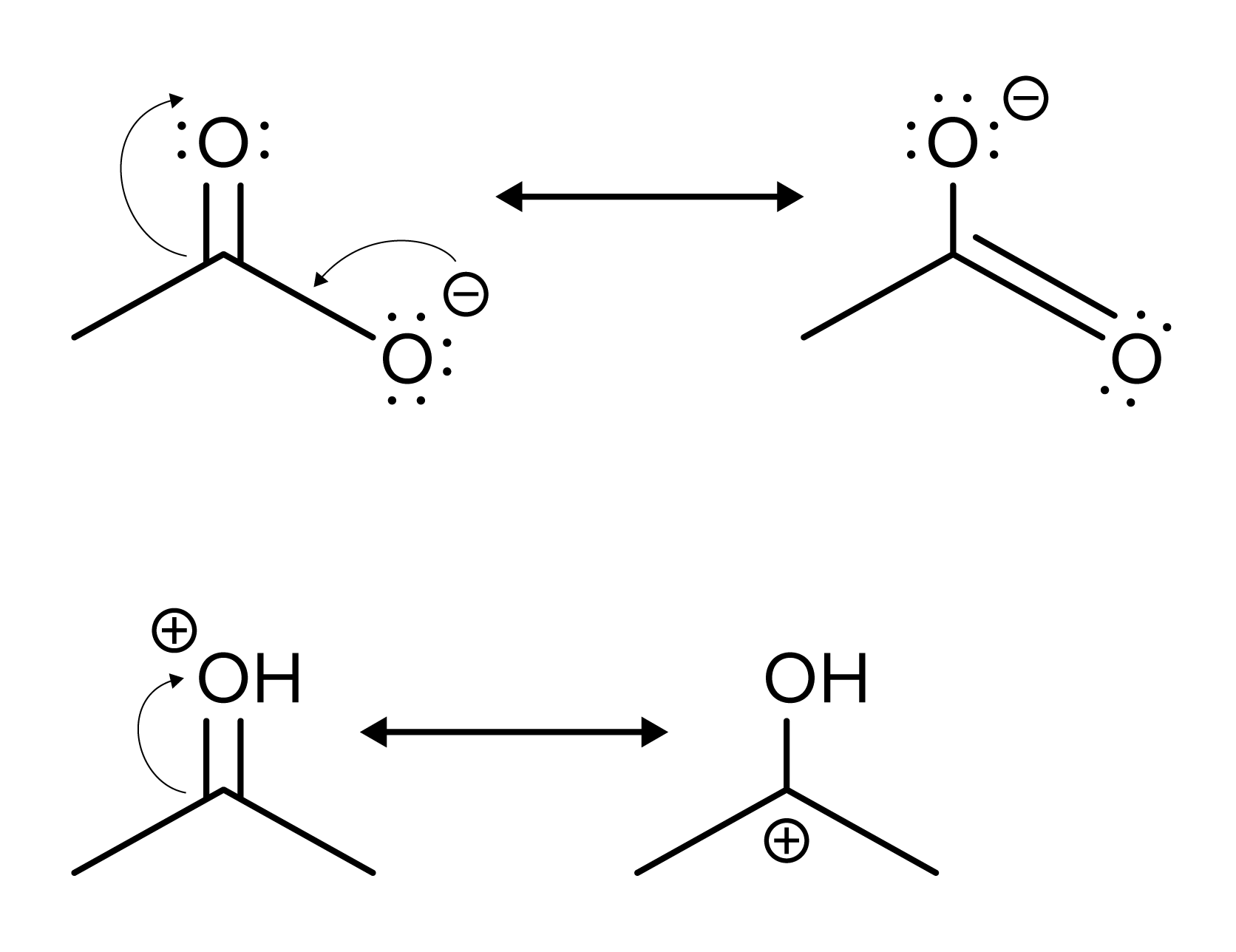
The DAT may ask you to determine what a resonance structure of a compound will look like. To do so, remember that negative charges push electrons and positive charges pull electrons. For example, a resonance structure for the top structures below pushes the electrons from the negative charge to create a double bond. The double bond to the other oxygen atom is subsequently pushed to the oxygen and its electrons are represented with a negative charge.
FIGURE 4: MOVING CHARGES ON RESONANCE STRUCTURES
Alternatively, the positive charge on the bottom compound pulls the electrons from the double bond towards it. As a result, there is a carbocation at the opposite end of where the double bond was attached.
If a single resonance structure doesn't actually show what the real molecule looks like, then how do we depict the real structure? Resonance hybrids are one way to show what the actual molecular structure looks like. In the resonance hybrid structure for ozone, the double bonds are shown as dashed lines in both locations that they resonate between. The positive charge is in the same spot for both resonance structures, so it is also depicted in the hybrid structure. To represent the negative charges though, a partial negative sign is placed on both the left and right oxygen atoms.
FIGURE 5: RESONANCE HYBRID STRUCTURE OF OZONE
For some compounds, resonance hybrids are not an even split of the resonance structures. A common DAT question asks you to identify which resonance structure contributes the most to the real structure of the compound. Whichever resonance structure is the most stable will be the greatest resonance contributor. To determine which structure is the most stable, look for structures that have:
Full octets
Lower formal charge
Negative charge on more electronegative atom
Positive charge on less electronegative atom
If resonance structures, like those for ozone, are not different in any of these criteria, then they are the same stability and contribute equally. Let’s go through some examples of these scenarios.
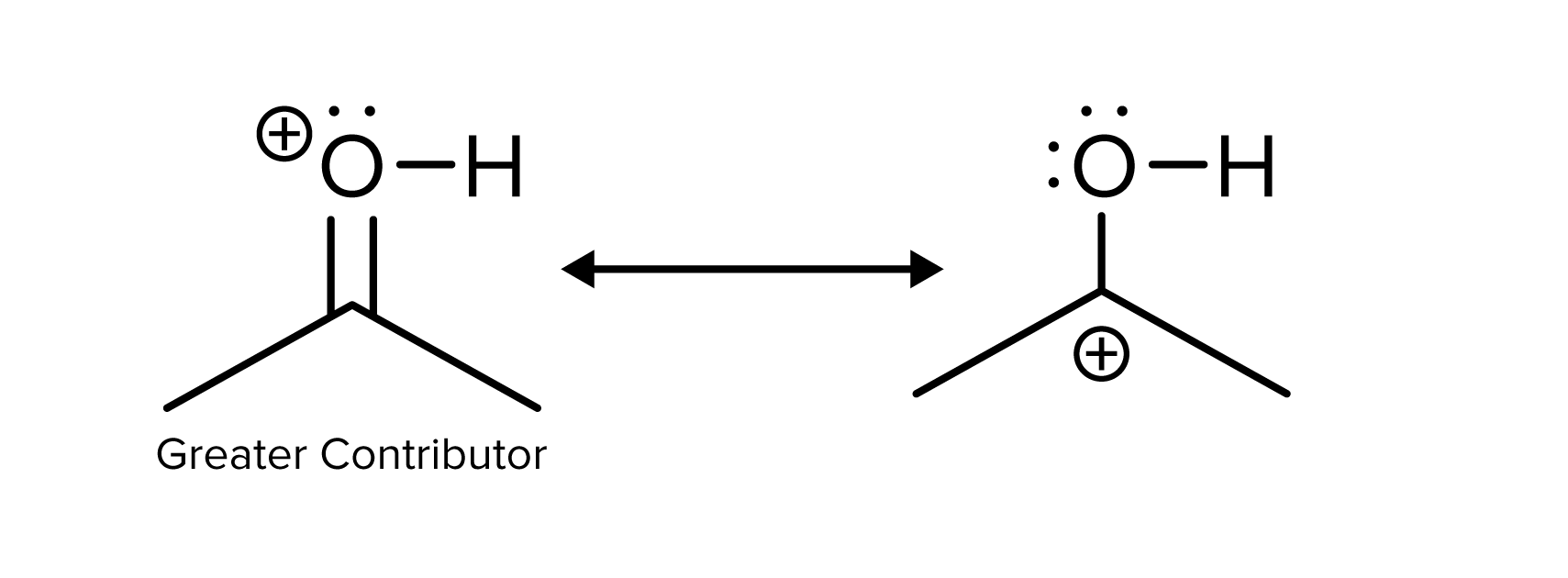
The compounds below are resonance structures, but the left structure contributes more to the real compound. The reason that it is a bigger contributor is that all atoms have a full octet. In the right compound, the positive charge is located on a carbon atom. This forms a carbocation that does not have a full octet, is not as stable, and does not contribute as much to the real form of the compound.
FIGURE 6: EFFECT OF OCTETS ON RESONANCE STABILITY
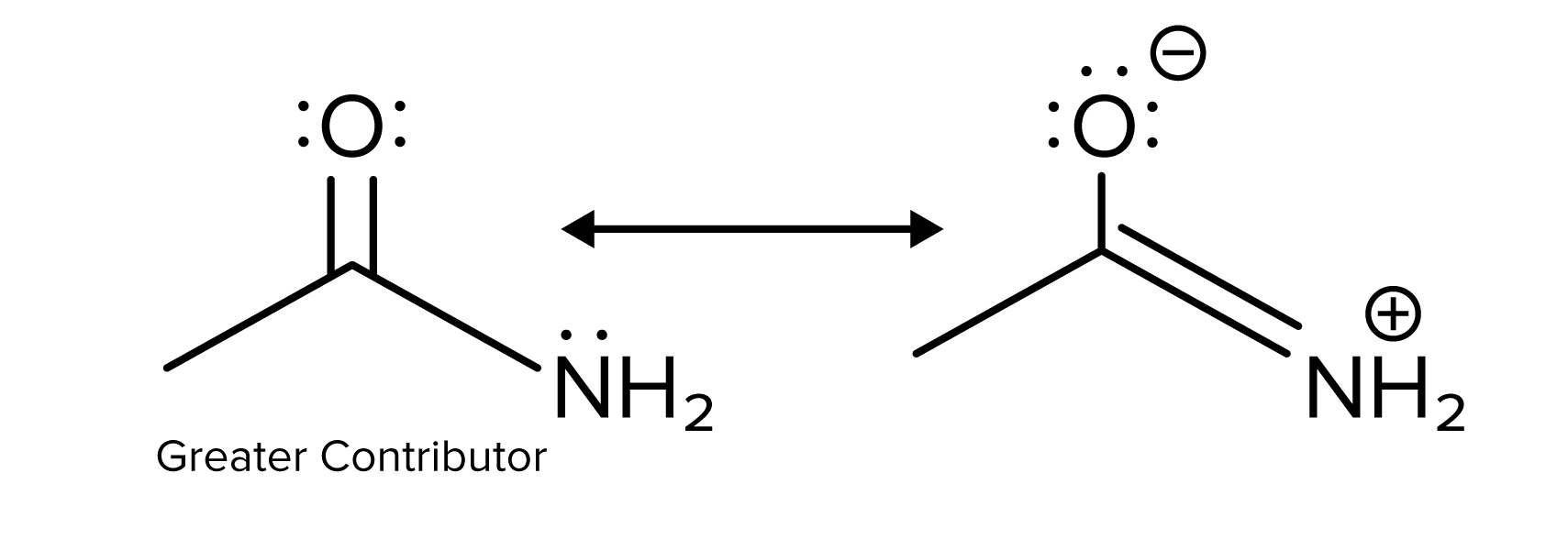
In this example, the left structure is again the greater contributor. The left structure does not have any charges, while the right structure has charges on both the oxygen and nitrogen atoms. Less formal charge means the structure is more stable, so the left structure is the greater resonance contributor.
FIGURE 7: EFFECT OF CHARGE ON RESONANCE STABILITY
Finally, in this example, the right structure is the greater resonance contributor. The difference between these resonance structures is the placement of the negative charge. Oxygen is more electronegative than carbon, so the resonance structure will be more stable if the negative charge is on the oxygen.
FIGURE 8: EFFECT OF CHARGE PLACEMENT ON RESONANCE STABILITY
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.