Acid-Base Organic Chemistry for the DAT
/Learn key DAT concepts about organic acids and bases, plus practice questions and answers
everything you need to know about acid-base organic chemistry for the dat
Table of Contents
Part 1: Introduction to acids and bases
Part 2: Organic acids and bases
a) Definitions
b) Strength
c) Identifying acidic protons
d) Predicting reaction products
Part 3: High-yield terms
Part 4: Questions and answers
----
Part 1: Introduction to acids and bases
Acids and bases are present in both nature and industrial processes. Understanding their properties and reactions is fundamental for studying organic chemistry and doing well on this section of the DAT. This guide provides an overview of how acids and bases affect organic reactions. Acid and base chemistry is tested in both the general and organic chemistry sections of the DAT. For a more general and comprehensive review of acids and bases, see our general chemistry guide on the topic.
----
Part 2: Organic acids and bases
a) Definitions
Recall that Brønsted-Lowry acids are compounds that donate protons (H⁺ ions), while Brønsted-Lowry bases accept protons. These types of acids and bases help make sense of reactions in aqueous solutions, where water often acts as the medium. Lewis acids are compounds that can accept an electron pair, while Lewis bases donate electron pairs. For the organic compounds covered in this guide, the Brønsted-Lowry definition will be used.
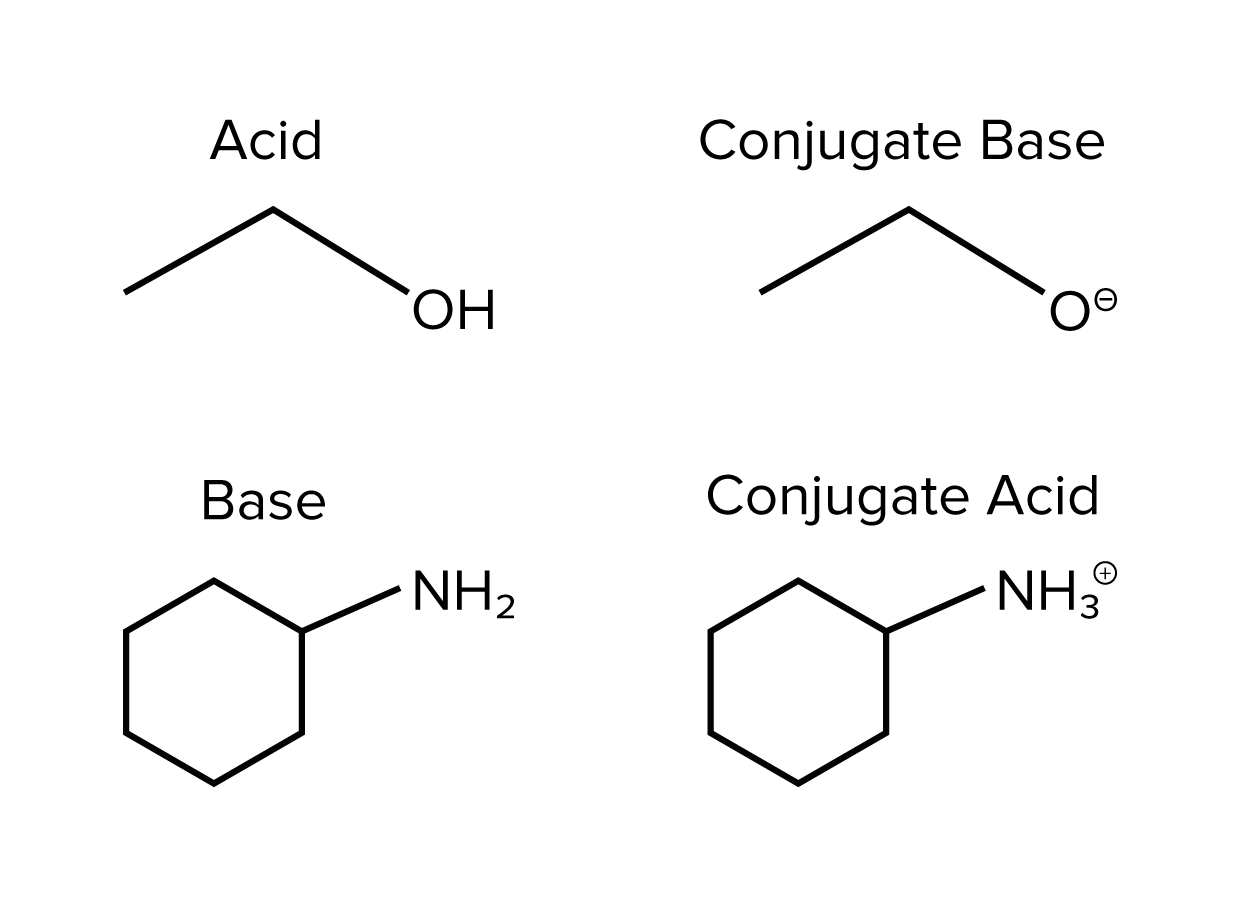
Acids and bases have conjugate bases and conjugate acids, respectively. In essence, each acid and conjugate base forms a pair, as does each base and conjugate acid. For any given acid, the conjugate base will be the same compound with one proton removed. An example of an acid/conjugate base pair is acetic acid, CH3COOH, and an acetate ion, CH3COO-. On the other hand, the conjugate acid of a base is simply the base with an added proton.
Here are examples of conjugate acid/base pairs.
FIGURE 1: ACID AND BASE WITH THEIR CONJUGATES
b) Strength
The structure of a compound is used to determine how strong or weak an acid or base is. Strong acids have low pKa values and weak conjugate bases, while weak acids have high pKa values and strong conjugate bases. A guiding principle is used to determine how strong or weak an acid is. This principle is that highly stable conjugate bases are weaker bases, meaning their associated acid is stronger. Less stable conjugate bases are stronger bases, which correlate to weaker acids. The same principle applies to conjugate acids; the more stable the conjugate acid, the weaker it is, and the stronger the base.
There are several factors that influence how strong or weak an acid or base is. Some of these factors require you to look at the stability of the conjugate base or conjugate acid, while others apply primarily to the non-conjugated acid or base. These factors include charge, atom, resonance, dipole induction, and orbitals. The mnemonic CARDIO is an easy way to remember these factors. Some of these factors look at the compound as a whole, while others focus on the acidic hydrogen atom. The acidic hydrogen atom, or acidic proton, is dissociated and donated from the acid.
Charge relates to any charges on the acid or base itself. Compounds that are more positively charged will be more acidic, while compounds that are more negatively charged will be more basic. This trend normally holds true when all compounds are relatively the same in all other factors.
FIGURE 2: EFFECT OF CHARGE ON ACIDITY
The atom that a hydrogen is attached to makes a difference in its acidity. Atoms further to the right on a periodic table and further down on the periodic table will have the most acidic hydrogen. So, acidity increases from left-to-right and top-to-bottom of the periodic table.
FIGURE 3: LARGER ATOMS AND ATOMS FURTHER TO THE RIGHT ON THE PERIODIC TABLE LEAD TO MORE ACIDIC PROTONS
Resonance of conjugate bases also plays a role in acidity. The ability of a compound to delocalize charge over a larger structure stabilizes the conjugate base, increasing acid strength. Looking at the acids and conjugate bases below, you can see that acids with conjugate bases that have resonance have a lower pKa, and therefore are more acidic.
FIGURE 4: RESONANCE IN CONJUGATE BASES INCREASES STABILITY, MAKING ACIDS MORE STABLE
Dipole induction is another factor influencing the strength of acids and bases. The presence of electronegative atoms nearby can stabilize the conjugate base by the inductive effect. Nearby electron-withdrawing groups increase acidity, while electron-donating groups decrease acidity. Recall that electron-withdrawing groups, such as a halide, are electron poor, while electron-donating groups like hydroxyl groups are electron rich. Additionally, the closer the electron-withdrawing group is to the acidic hydrogen, the stronger the acid is.
FIGURE 5: ELECTRONEGATIVE ATOMS AND PROXIMITY INCREASE ACIDITY
Orbital hybridization is the final factor to consider. Acidity increases as the s-character of the hybrid orbital holding the acidic hydrogen increases (sp > sp2 > sp3). This is because s-orbitals are closer to an atom’s nucleus and thus closer to its protons.
FIGURE 6: ORBITAL HYBRIDIZATION AND ACIDITY
----
Gain instant access to the most digestible and comprehensive DAT content resources available. Subscribe today to lock in the current investments, which will be increasing in the future for new subscribers.